Pharmaceutical Company Which Makes Fentanyl Running CBD Clinical Trials
- Insys Therapeutics, makers of Fentanyl, is running clinical trials into medical properties of cannabidiol (CBD)
- Insys donated $500,000 last year to the campaign to defeat cannabis legalization in Arizona
- The US is currently fighting an opioid epidemic, which claimed the lives of more than 15,000 people in 2015
A large pharmaceutical company which spent half a million dollars campaigning against cannabis legalisation in Arizona is now running clinical trials into cannabidiol (CBD).
Insys Therapeutics, makers of synthetic opioid Fentanyl, is currently running a number of clinical trials investigating the use of cannabis-based therapies for a variety of ailments.
Some of the trials they are currently running are: “Cannabidiol Oral Solution in Pediatric Participants With Treatment-resistant Seizure Disorders,” (medication for treatment-resistant child epilepsy) “Cannabidiol Oral Solution for Treatment of Refractory Infantile Spasms,” and “Cannabidiol Oral Solution for The Treatment of Subjects With Prader-Willi Syndrome.”
Other pharmaceutical companies have already funded research into CBD, such as GW Pharmaceuticals research into treatment-resistant epilepsy. Why would a company which spent so much money lobbying against the legalisation of medical marijuana start researching the very chemicals it deems so harmful?
America’s current opioid epidemic may hold the answer.
Opioids (including prescription opioids, heroin, and fentanyl) killed more than 33,000 people in 2015, with more than 15,000 people dying from overdoses involving prescription opioids.
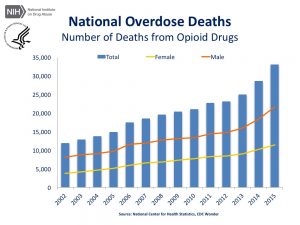
Medical Marijuana has long been seen as the answer to this growing opioid crisis.
Cannabis is a threat to legal pharmaceutical opioid-based medications as it is able to deal with the chronic pain which most people are prescribed opioids for. A recent study found that 97% of patients “strongly agreed” that they were able to decrease the number of opiates they consume when they also used cannabis.
Cannabis is so successful at replacing these dangerous opioid medications that opioid deaths fell by 6.5% two years after Colorado legalised cannabis for recreational purposes in 2015.
But why would a company, which can clearly see the potential medical properties of cannabinoids, spend so much money on keeping the very plant they come from criminalized? Money.
Companies such as Insys and GW stand to earn a significant amount of money if patients start using cannabinoid-based medication if cannabis, the plant, is kept illegal.
Patients will be forced to source their medication for large pharmaceutical companies rather than be able to grow their own, at a fraction of the cost.
Sativex, the UK’s only legal cannabinoid-medication, is currently prescribed on the NHS at a cost of £125 per 10ml bottle, or up to £5500 per year. For many patients, this is a cost which cannot be met.
Pharmaceutically made cannabis may be a great option for some patients, but it should not be their only choice. There is a fantastic range fo methods of medicating with cannabis, including ingesting it as an “edible.”
If we remove the patient’s right to chose their own medication we are limiting their quality of life.
Have you replaced your pharmaceutical medications with cannabis? Let us know in the comments.[/fusion_text][/three_fourth][fusion_text]
References and further Reading
https://clinicaltrials.gov/ct2/show/NCT02324673?term=Insys+cannabidiol&rank=1
https://clinicaltrials.gov/ct2/show/NCT02551731?term=Insys&draw=1&rank=10
https://clinicaltrials.gov/ct2/show/NCT02844933?term=Insys&draw=1&rank=5
