UK Charity launches medical cannabis oil trial to help treat rare skin condition
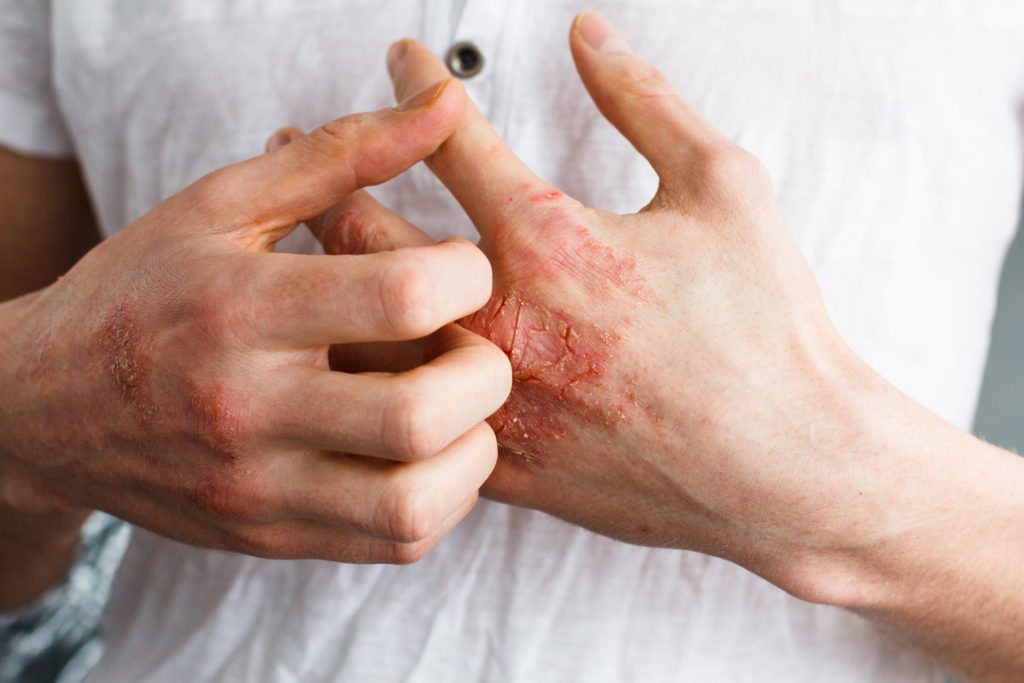
- A trial has been launched to see if cannabis can help treat Epidermolysis Bullosa (EB), which causes the skin to easily blister and tear
- The study will take place in the Netherlands, where cannabis research laws are less restrictive
- EB affects more than 5,000 people in the UK, causing pain and itching, with blisters having to be drained and dressed every day
- Only adults from the Netherlands have been allowed to participate
A British charity has launched a medical study to see if cannabinoid-based medicines (CBMs) can help treat a rare skin condition.
The trial was launched by DEBRA, the only British charity which supports people who suffer from Epidermolysis Bullosa (EB), at the end of 2017.
EB causes the skin to tear and blister at the faintest touch and is reportedly very painful to deal with. Currently, the only available treatments in the UK include a prolonged used of opioid-based pharmaceuticals and anti-inflammatories, which are infamous for their dangerous side effects.
The study is investigating fifteen participants from the Netherlands, aged 18 and over, over a three-year period at the Centre for Blistering Diseases at the University Medical Centre Groningen in the Netherlands.

The treatment being followed in this trial is a CBM oil, which contains naturally derived phytocannabinoids from the cannabis plant.
The trial will only be investigating a very weak cannabis oil (CBM), with only 1.3% THC and 2% CBD. Other phytocannabinoids will be present in the cannabis oil, but in smaller quantities.
Participants in the study will use pipettes to drop the CBM under their sublingual gland (under the tongue), four times a day (after breakfast, lunch, dinner and before sleeping).
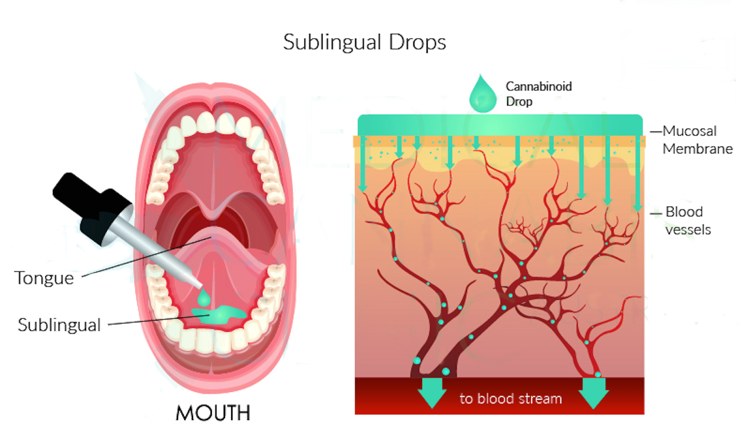
The sublingual gland is the thinnest layer of skin in the body, allowing cannabinoids to enter the bloodstream where they are absorbed faster. This will minimise each individual’s metabolism of cannabinoids, reducing the possibility of side effects.
“Anecdotal reports from people suffering from EB suggest that cannabinoid-based medicines (CBMs) are effective for pain and itch symptom control.”
– Caroline Collins, director of research at DEBRA
]In a press release, DEBRA explained why they were funding this clinical trial on medical cannabis:
“This research is a very important first step in gathering the scientific evidence needed to prove that a cannabis-based medicine could be a safe and effective form of pain relief for EB and ultimately making an effective form of cannabis-based pain relief available to people suffering from EB.”
Anecdotal evidence from EB sufferers inspired the investigation:
“Members of the EB Community in many countries around the world have widely reported that cannabis can reduce the impact of pain and itch caused by EB.
“This trial is vital to help find the safest and healthiest way to deliver medicinal cannabinoids to people with EB, with the maximum alleviation of symptoms and the minimum of side effects.”
The trial already has had initial success, proving that cannabinoid-based medicines may indeed be a better way to treat EB than opioids and anti-inflammatories.
The first three patients recruited to the trial have already reported improved pain scores, reduced itching (pruritus) and reduction in overall pain medication intake, although the researchers stress that further research is needed.
Discussing the decision to fund the trial, Caroline Collins, director of research at DEBRA, said:
“We are extremely pleased to fund this clinical trial, particularly in light of the government’s decision to legalise some forms of medical cannabis.
“Anecdotal reports from people suffering from EB suggest that cannabinoid-based medicines (CBMs) are effective for pain and itch symptom control.
“The clinical trial will start to gather the scientific evidence needed to prove that CBMs are an effective treatment for pain and itch caused by EB and will begin the work towards a new treatment protocol and evidence-based guidelines for the management of these debilitating symptoms, which we hope will improve the quality of life for many.”
DEBRA’s President, Simon Weston CBE, commented on why this research was of a special, personal, importance to him:
“For many years, I was taking opiates to deal with the pain from both injuries and surgery.
“An alternative method of pain control will be a welcome relief.”
