Evidence for Cancer “Committing Suicide By Eating Itself” After Exposure to Cannabis
Evidence for Cancer “Committing Suicide By Eating Itself” After Exposure to Cannabis
The American Association for Cancer Research presented the results of a study carried out at Harvard University in 2007 and this still remains the most comprehensive ever realised on THC’s potential to combat tumour growth.
The study indicated that THC has both anti-tumorigenic and anti-metastatic effects on lung cancer cells. The authors found that doses of THC were able to cut lung cancer tumour growth in murine subjects in half in just three weeks and to reduce cancer lesions by even more.
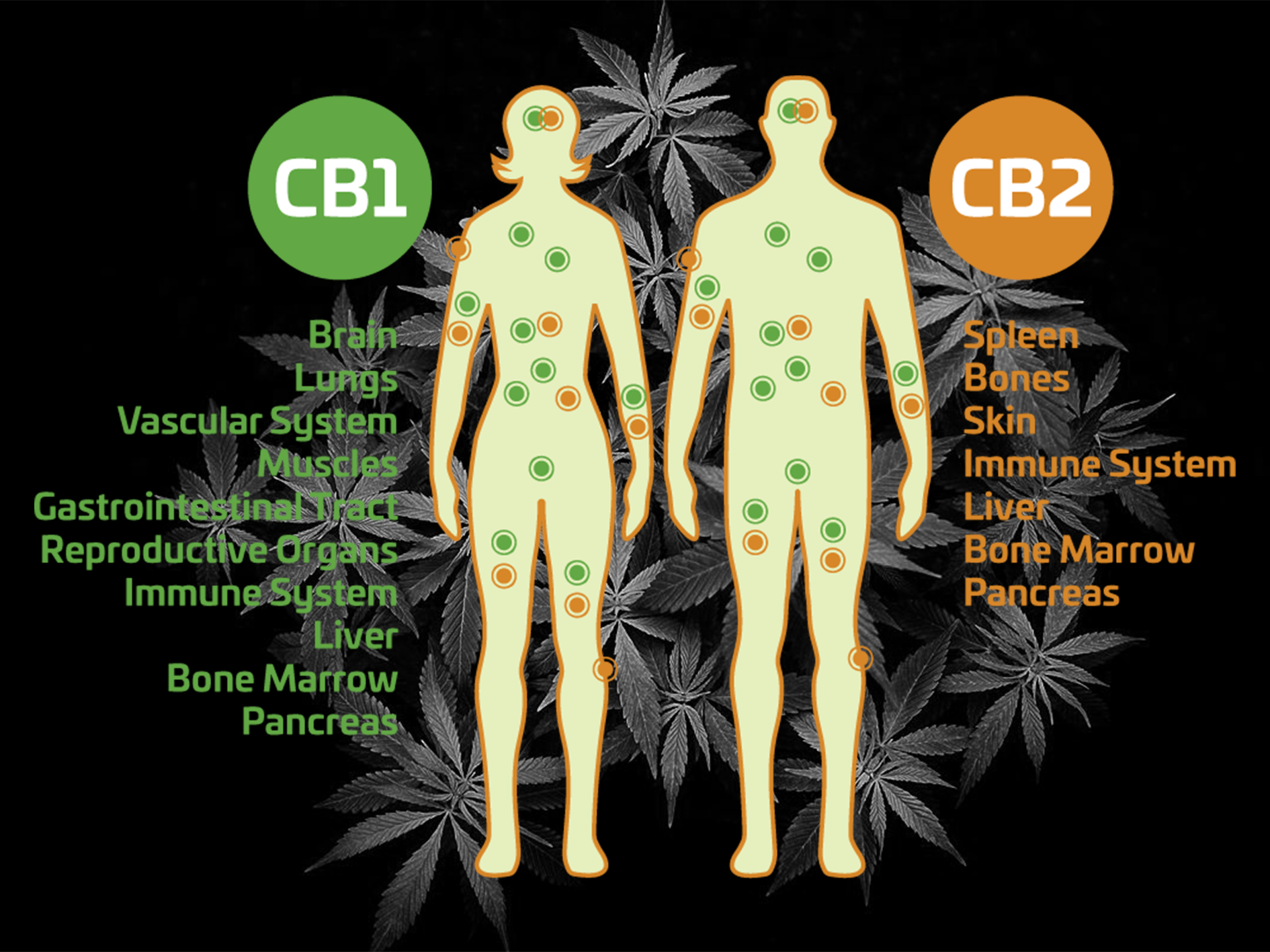
THC, that targets cannabinoid receptors CB1 and CB2, is similar in function to the endocannabinoids which are naturally produced in the body and activates these receptors. The researchers suggest that THC and other “designer agents “ that activate these receptors might be used in a targeted fashion to treat lung cancer. “The beauty of this study is that we are showing that a substance of abuse, if used prudently, may offer a new road to therapy against lung cancer,” commented Anju Preet, Ph.D., a researcher in the Division of Experimental Medicine at the university.
Acting through cannabinoid receptors CB1 and CB2, endocannabinoids (as well as THC) are thought to play a role in variety of biological functions, including pain and anxiety control as well as the modification of inflammatory processes.
In the present study, the researchers first demonstrated that two different lung cancer cell lines as well as patient lung tumour samples have CB1 and CB2 receptors on the surface of the cells, and that non-toxic doses of THC inhibited growth and spread in the cell lines.
Lung cancers that over-express EGFR are usually highly aggressive and resistant to chemotherapy treatments. “When the cells are pre-treated with THC, they have less EGFR-stimulated invasion as measured by various in-vitro assays,” Preet said in 2007.
Why 13 years later on has so little attention been given to this potentially revolutionary therapeutic?
Pascalbiosciences, an American pharmaceutical company, seems to think there is money in such research.
Researchers injected standard doses of THC during three weeks into mice that had been implanted with human lung cancer cells and found that tumours were reduced in size and weight by about 50 percent in the treated animals compared to a control group. Additionally there was a 60 percent reduction in cancer lesions on the lungs of these mice as well as a significant reduction in protein markers associated with cancer progression.
Although the researchers do not know why THC inhibits tumour growth, they hypothesise that it could be activating molecules that can arrest the cell cycle. They speculate that THC may also interfere with both angiogenesis, a process through which new blood vessels form from pre-existing vessels, and vascularisation, which is often deemed the holy grail of tissue engineering because it is one of the key preconditions that determine the in-vivo viability of tissue constructs. Both these processes promote cancer growth and by disrupting them THC slows the growth of the cancer down to such an extent that it is no longer being supplied with the requisite growth conditions. More work is needed to clarify the mechanism by which THC functions, and on a cautionary note other unrelated animal studies have shown that THC can stimulate the growth of some cancers and in particular hormone-driven breast cancers .
THC offers significant promise, but there is a long way to go before its full potential can be identified and then realised.
The research was presented at the 2007 meeting of the American Association for Cancer Research, held Apr 14-18, 2007 in Los Angeles, CA. Why 13 years later on has so little attention been given to this potentially revolutionary therapeutic?
Pascalbiosciences, an American pharmaceutical company, seems to think there is money in such research:
https://www.pascalbiosciences.com/
New research finds more evidence medical cannabis may reduce seizures in epileptic children
New research finds more evidence medical cannabis may reduce seizures in epileptic children
According to a phase 3 study released today that will be presented at the American Academy of Neurology’s 71st Annual Meeting in Philadelphia, May 4 to 10, 2019, taking a pharmaceutical formulation of cannabidiol cut seizure rates nearly in half for children with a rare and severe type of epilepsy. This is called Dravet syndrome, which starts in infancy, and can lead to intellectual disability and frequent, prolonged seizures. Cannabidiol [CBD] is derived from marijuana that does not include THC, the psychoactive part of the plant that creates a so-called “high.”
“It’s exciting to be able to offer another alternative for children with this debilitating form of epilepsy and their families,” said study author Ian Miller, MD, of Nicklaus Children’s Hospital, [formerly Miami Children’s Hospital] in Florida. “The children in this study had already tried an average of four epilepsy drugs with no beneficial effect and at the time were taking on average three additional drugs, so to have this measure of success with cannabidiol is a major victory.”
The study involved 199 children with an average age of 9, who were divided into three groups of equal size. One group received 20 milligrams per kilogram (mg/kg) per day of cannabidiol, the second group received 10 mg/kg per day and the third group received a placebo.
“It’s exciting to be able to offer another alternative for children with this debilitating form of epilepsy and their families.”
– Ian Miller, Study Author
In order to establish a comparative baseline the number of seizures was recorded for four weeks before the treatments were started. The participants then received the treatment for 14 weeks. By the end of the study, those taking the high dose of the drug showed a decrease in seizures with convulsions by 46 percent and by 49 percent for those taking the lower dose of the drug. In comparison, those taking the placebo showed a reduction in the number of seizures of 27%.
The number of total seizures reduced by 47 percent for those in the high dose group, by 56 percent for those in the lower dose group and by 30 percent for those in the placebo group. In the high dose group, 49 percent of the participants had their seizures cut in half or more, compared to 44 percent in the low dose group and 26 percent in the placebo group.
All of the groups reported side effects, including 90 percent of the high dose group, 88 percent of the low dose group and 89 percent of the placebo group. The most common side effects were decreased appetite, diarrhea, sleepiness, fever and fatigue. About 25 percent of those in the high dose group had serious side effects, compared to 20 percent of those in the low dose group and 15 percent of those in the placebo group. Only participants in the high dose group, numbering 7% in total stopped taking the drug as a result of these side effects. “Based on these results, dose levels above 10 mg/kg per day should be carefully considered based on the effectiveness and safety for each individual,” Miller said.
This article has been republished from materials provided by the American Academy of Neurology. Note: material may have been edited for length and content. For further information, please contact the cited source.
Decoding the Secrets of the Endocannabinoid System
Decoding the Secrets of the Endocannabinoid System
There is currently a debate in the UK there on the impact of medical cannabis and cannabinoids.
Though this discussion is important, it is overlooking the real medical marvel that is the endocannabinoid system and the significant therapeutic potential that the Endocannabinoid System (ECS) offers.
Prior to the synthesis and identification of Tetrahydrocannabinol (THC) in 1964, the scientific community had no idea how or why cannabis worked.
Initially, it was thought that cannabis compounds, such as THC or Cannabidiol (CBD), acted in a non-specific manner attaching to the outer walls of human cells. Little did we know that in 1988 we would discover the tip of the iceberg, the Cannabinoid receptor 1 (CB1).
The discovery of this novel receptor prompted the investigation into the internal molecules mediating the function of CB1 in the human body.
Studies by American researcher Howlett, et al, in 1990, and by Pacher, Bátkai, and Kunos in 2006, identified the endogenous molecules now known as endocannabinoids.
The molecular cloning of the cannabinoid receptor 1 in 1990 by Matsuda, et al, gave scientists the ability to produce synthetically compounds that could specifically target cannabinoid receptors. Receptors in the body act much like a light switch that can be turned on and off.
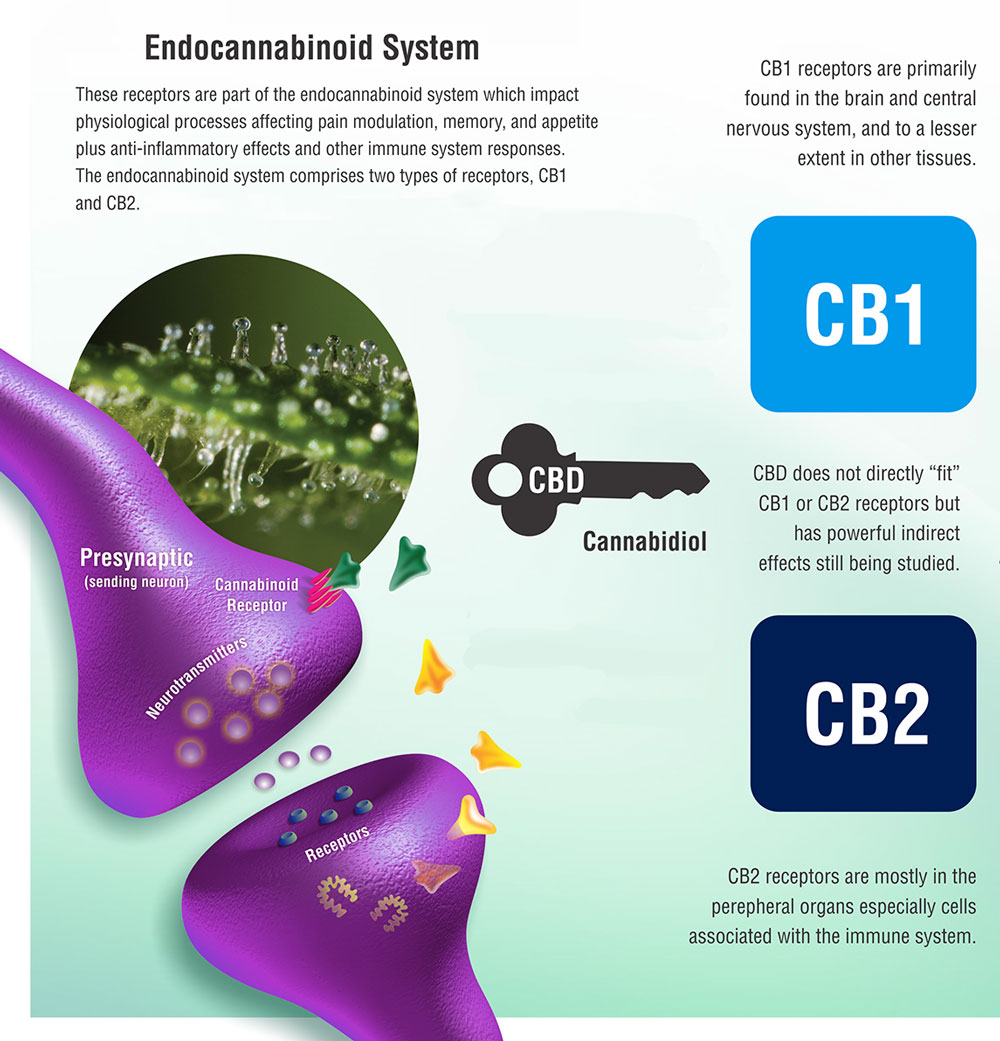
Using pharmaceutically engineered molecules researchers were able to use a process of elimination by activating and deactivating receptors in to explore their effects.
This seemingly insignificant discovery has led to the identification of an entirely new and previously undiscovered regulatory system, the Endocannabinoid system (ECS).
The discovery of this system promises to change our entire understanding of disease and has given a new impetus to 21st-century medicine.
Though our understanding of the ECS is still in its infancy, it is already abundantly clear to scientists that this intricate regulatory system controls the activity of all cells within the body and in principle has the potential to revolutionize our interpretation of health and disease.
So what is the endocannabinoid system?
Initially, it was thought it comprised only two receptors (cell light switches) the Cannabinoid Receptor 1 and 2 (CB1 and CB2). This could not have been further from the truth.
Little did we know, the human body produced its own molecules, now known as endocannabinoids, that interact with these cannabinoid receptors so as to regulate the functionality and health of individual cells.
These internal endocannabinoid molecules that naturally occur universally within all mammals are chemically identical to the phytocannabinoids such as THC and CBD that are found in the Cannabis plant.
Interestingly, we only discovered these internal molecules as the result of efforts to understand why cannabis produced psychoactive effects.
The realisation that cannabinoids existed in the human body enabled us to provide a clear picture of the main elements of the endocannabinoid system.
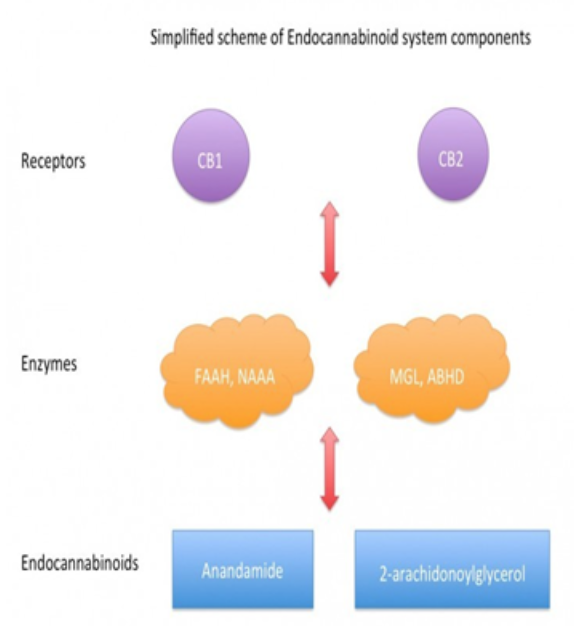
This system is made up of three simple components.
Firstly, the naturally occurring endocannabinoids are synthesized when needed and digested when no longer required. This process of synthesis and digestion is performed instantaneously by a specific set of enzymes that control the quantities of endocannabinoids within the body.
Enzymes are found throughout the body and act as a biological machine that facilitates the vital chemical reactions that take place constantly throughout the body. Enzymes in the gut allow the body to digest nutrients and food and convert them into molecules that the body can use.
Similarly, we have enzymes that specifically breakdown endocannabinoids into smaller molecules and reproduce them when they are needed. These enzymes regulate the quantities of endocannabinoids in the body which in turn alters the strength and regularity of signals received by the receptors of the endocannabinoid system.
When an endocannabinoid activates a cannabinoid receptor a variety of chemical changes occur within those cells and tissue and results in profound effects on features such as appetite, mood, blood pressure, and the immune system.
Every process within the body is controlled in some way by these three components of the endocannabinoid system and for this reason, we often see that this coordinated regulation is damaged or becomes faulty in some way during disease states.
The ratios of these three components are constantly changing dynamically and are altered by the body according to our specific needs.
“More importantly, modulating the activity of the endocannabinoid system turned out to hold therapeutic promise in a wide range of disparate diseases and pathological conditions, ranging from mood and anxiety disorders, movement disorders such as Parkinson’s and Huntington’s disease, neuropathic pain, multiple sclerosis and spinal cord injury, to cancer, atherosclerosis, myocardial infarction, stroke, hypertension, glaucoma, obesity/metabolic syndrome, and osteoporosis, to name just a few.“
– Pacher, et al, 2006.
Our knowledge of the underlying principles of this system provides us with an opportunity to utilise eventually this intricately balanced process for specific therapeutic purposes.
This balance is almost always compromised in most diseases causing the body to lose control of its regulatory processes.
The importance of this cannot be understated.
The human body is an intricate machine that is constantly making minute molecular changes to maintain a carefully balanced and equilibrated state known as homeostasis.
This same principle applies to the ECS.
In healthy humans, the endocannabinoid tightly regulates the bodies many systems maintaining a smooth-running “well-oiled “machine.
In disease states, such as cancer, for example, the intricate regulatory system that is the endocannabinoid system is disrupted and resulting in an unbalanced regulatory process.
In colorectal cancer, for example, studies have demonstrated that colorectal cancer tumor cells have fewer CB1 receptors and overproduce CB2 receptors.
Further studies have demonstrated that these tumour cells, with their altered expression, respond positively to cannabis-based therapeutics, though we are far from understanding why. As yet we are merely exploring the potential of these findings but as our understanding develops, we will one day decode unravel the puzzle that is the endocannabinoid system.
As our knowledge matures, we will develop the ability to target and manipulate the endocannabinoid system to the therapeutic benefit of millions of people.
This key system has the potential to reshape medicine as we know it allowing the invasiveness of medical treatments to be reduced. The significance of the ECS is that one day it may be possible to utilise the endocannabinoid system as a medical tool to manipulate the body into treating itself, removing the need for invasive surgical procedures and highly toxic treatments.
In a manner similar to that used by a computer technician to re-programme PC software to make it quicker and more user-friendly, we will one day be able to decode a person’s endocannabinoid system and manipulate it to alleviate suffering.
If you are fortunate enough to take part in some form of medical cannabis debate or discussion, then speak carefully but knowledgeably of the ECS and consider for a moment the potential it has to change one’s life or that of a loved one.
References and further Reading
Howlett, A. C. et al. (1990) ‘The cannabinoid receptor: biochemical, anatomical and behavioral characterization.’, Trends in Neurosciences, 13(10), pp. 420–3. Available at: http://www.ncbi.nlm.nih.gov/pubmed/1700516 (Accessed: 16 September 2018).
Pacher, P., Bátkai, S. and Kunos, G. (2006) ‘The Endocannabinoid System as an Emerging Target of Pharmacotherapy’, Pharmacological Reviews, 58(3), pp. 389–462. doi: 10.1124/pr.58.3.2.
What are the major barriers to the wider use of hemp-based plastics?
What are the major barriers to the wider use of hemp-based plastics?
Plastics are an essential part of our everyday lives but their future is compromised by two factors: the continuing availability and use of carbon-based, raw materials comprising non-renewable gaseous or liquid petrochemicals and secondly the disposal of plastics at the end of their useful life.
Not only are the harmful effects of global warming increasingly clear, but alternative biodegradable materials are needed as waste plastics pollute both water and land.
To make matters worse conventional plastics appear to linger in the environment and can even enter the food chain with a resultant detrimental effect on both human and animal health.

The oceans are in a poor state of health, thanks to the presence of so-called “microplastics”, [tiny fragments of plastic waste] that pollute the waters and are often consumed by marine life.
The infamous “Great Pacific Garbage Patch” is actually composed of millions of these tiny particles.
As much as 1.9 million items per square mile, according to a report by National Geographic.
In one of the most widely quoted articles, the details of which are especially shocking, research scientists from the University of Tasmania in Australia and the UK’s Royal Society for the Protection of Birds found 38 million pieces of plastic waste on Henderson Island, an uninhabited coral island in the South Pacific. Jennifer Lavers, a marine scientist at the University of Tasmania, commented:
“I’ve travelled to some of the most far-flung islands in the world and regardless of where I’ve gone, in what year, and in what area of the ocean, the story is generally the same: the beaches are littered with plastic waste, clear evidence of human activity.”
To underline still further this point, a recent Ellen MacArthur Foundation study forecast that plastic waste particles could outnumber fish in the ocean by 2050. Can we really afford to let this happen?

What are the alternatives to petrochemicals as a resource for plastics production?
You may be aware that agricultural hemp, an organic plant which is the “non-mind-altering” cousin of cannabis (commonly known as marijuana), has dozens of potential uses ranging from clothing via building materials to paper. Some of the earliest plastics in commercial use such as cellophane, rayon, and celluloid were made from cellulose fibres obtained from hemp which contains around 65-70% cellulose.
This can be compared with wood, which contains around 40% cellulose, flax 65-75% cellulose, and cotton with up to 90% cellulose. The particular promise of hemp was based on its relative sustainability and low environmental impact.

As early as 1941 Henry Ford had made a motor car with hemp fibre reinforced body panels with an engine running on plant-based liquid fuels.
Unfortunately, this was not a commercial success in part as a result of the Second World War. Since virtually all climate scientists agree that we must replace or at least significantly reduce our dependence on fossil fuels, and given that hemp can even make the soil cleaner, it is somewhat surprising that this miracle crop is not in wider use. Are there any basic reasons for this?
Though hemp requires fewer pesticides and has a smaller environmental footprint than many other crops, growing and harvesting it remains labour intensive but this does give the manufacturers of mechanised equipment the attractive possibility of designing and producing more efficient alternatives to manual methods.
Another drawback is that hemp, requires significant fertiliser in some soils, and also has relatively high water requirements.
While a 100% hemp-based plastic is still a rarity, some “composite bioplastics”, which are made from a combination of hemp and other fibrous plant sources, are already in use.

Thanks to their high specific strength [when measured as a function of density] and stiffness, these plastics are currently used as structural components in cars, boats, and even musical instruments.
One of the most significant examples of hemp’s future as a potential raw material for making plastics could come from LEGO, the ubiquitous building block toy company based in Denmark, which is promising to phase out the use of fossil-fuel based resins by 2030.
Could hemp be the cost effective, environmentally sustainable alternative material?
The most significant barrier to wider use of hemp however is essentially a social issue and results from the so-called US “war on drugs”, which historically has resulted in many restrictions being placed on legally growing hemp and cannabis.
Putting an end to this “war” would have a profound effect on the use of hemp as production would be legalised and distribution widespread. Improved mechanised facilities would reduce the labour element in costs and further.
Cannabis based drugs: how will they be used in practice in the UK?
Cannabis based drugs: how will they be used in practice in the UK?
- Medical cannabis was legalised for doctors to prescribe on 1 November
- But who will be able to issue these products, for what conditions, and what’s the evidence?
- In this article, we will provide a clear guide to the new regulations and how it will affect patients in Britain who want to access medical cannabis products
Under the new guidelines, published by NHS England last week, only specialists will be able to prescribe cannabis-based drugs, and they will be expected to get approval from the chair of their hospital’s drug and therapeutics committee, or the medical director, on a named patient basis.
GPs alone will not have the ability to prescribe medical cannabis, you will need a full team of approval before being granted access.
What medical conditions will qualify under the new guidelines?
Currently, just two are listed by the NHS: children with rare forms of epilepsy and adults with nausea or vomiting induced by chemotherapy.
The guidelines themselves outline the very limited scope of patients who will be able to access medical cannabinoids:
“Very few people in England are likely to get a prescription for medical cannabis.”
What’s the evidence of benefit in these conditions?
Deb Pal, professor of paediatric epilepsy at the Institute of Psychiatry, Psychology and Neuroscience at King’s College London, says that there is now good evidence from clinical trials that drugs based on cannabidiol are effective against two types of severe childhood epilepsy: Dravet syndrome and Lennox-Gastaut syndrome.
Epidiolex, an oral solution of cannabidiol, has been licensed in the US for these two indications but not yet in Europe.
There is also good evidence that cannabinoids are effective in preventing nausea and vomiting induced by chemotherapy, say recommendations from the Royal College of Physicians.
But more effective treatments are available, the RCP says, and cannabinoids should be used only by patients for whom standard treatments have failed.
“Very few people in England are likely to get a prescription for medical cannabis.”
– NHS England
Are any cannabis-based drugs licensed in the UK?
Nabiximols (sold in the UK as Sativex) has been licensed since 2010 as a treatment for spasticity in multiple sclerosis. It contains cannabidiol and tetrahydrocannabinol (THC).
The National Institute for Health and Care Excellence, however, says that it is not cost effective and should not be used in the NHS.
Who makes cannabis products?
Sativex and Epidiolex are made by the British company GW Pharmaceuticals, which is based in Cambridge. The company is supplied with cannabis by British Sugar, which grows it in a huge glasshouse at Wissington, near Downham Market in Norfolk.
What’s the evidence of harm?
Epidiolex trials showed side effects such as sleepiness, sedation, and lethargy; elevated liver enzymes; decreased appetite; diarrhoea; rash; fatigue, malaise, and weakness; insomnia, sleep disorder, poor quality sleep, and infections.
Drugs that include THC have additional risks. The Royal College of Physicians lists psychosis, dependency, hallucination, and suicidal thoughts as possible side effects.
The Royal College of Paediatrics and Child Health warns that THC may affect the developing brain and cause alterations to IQ and mental health.
However, much uncertainty remains because the legal status of cannabis has hitherto discouraged research. Can private GPs prescribe these products?
No.
Under amendments to the Misuse of Drugs Regulations only “specialist medical practitioners”, defined as those listed on the General Medical Council’s specialist register, are permitted to prescribe unlicensed cannabis-based products.
Anybody else who did so would be breaking the law.
What should GPs do if a patient asks for one of these products?
GPs should explain that they cannot prescribe the drugs.
If they believe that the patient may meet the very narrow definitions laid down by NHS England, which include the lack of any licensed product that would meet the patient’s needs, they could refer the patient to a specialist.
Many people claim cannabis eases chronic pain. Are they excluded?
Yes.
NHS England says that the evidence is insufficient to recommend cannabis-based drugs for pain and that, lacking such evidence, they should not be prescribed.
The Royal College of Physicians says that there has been limited evidence of effectiveness in some studies of palliative care patients but notin others.
Results of Sativex trials in this indication are equivocal. For long-term neuropathic pain, a Cochrane review in March 2018 concluded that potential benefits were outweighed by the risks, and a systematic review published in 2015 reached the same conclusion.
A disappointing outcome?
For patients with chronic pain, yes, especially as the review by England’s chief medical officer that underpinned the recent law change cited among its “conclusive or substantial” evidence claims that cannabis-based medicines were effective for chronic pain.
Genevieve Edwards of the MS Society said:
“The guidance published appears to ignore the clear evidence provided by the chief medical officer. For this change to have a real impact, everyone who could benefit must be given access in a safe, responsible, and fair way, with specialist doctors properly supported to make decisions around prescribing.”
Does the legal change go too far or not far enough?
Taken together, the change in the status of cannabis-based medicines and the NHS England rules represent a minimalist…
References and further Reading
Cannabis and Chronic Pain: How CBD Can Help Manage Pain
Cannabis and Chronic Pain: How CBD Can Help Manage Pain
- Cannabidiol (CBD) has been reported by many new users to help manage their chronic pain
- We look into the “new health craze” to find out how it is helping patients both with their symptoms and their addictions to dangerous pharmaceutical medications
Words by Jessica Jones
Opioid abuse and overdose claimed an estimated 64,000 lives in 2016 alone.
President Donald Trump has recently declared opioid epidemic as a public health emergency and pointed it as the worst drug crisis in the national history.
This crisis raises an urgent need to find an effective solution to the addiction.
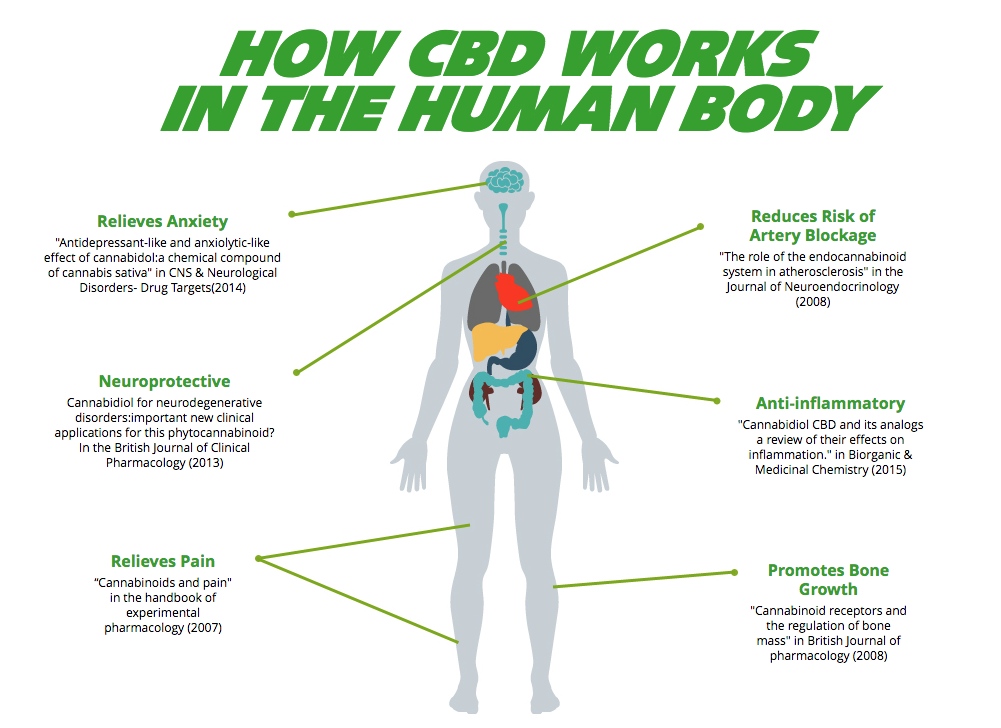
Research into cannabidiol (CBD) has shown a lot of potential to be used as a therapy for many addictions as well as for treatment of various disorders and chronic diseases including; cancer, nausea, diabetes, cardiovascular diseases and depression, without exerting long-term side effects.
Studies have shown that it can be helpful in counteracting the effect of opioid and alleviating withdrawal symptoms like anxiety, pain and nausea.
What is CBD?
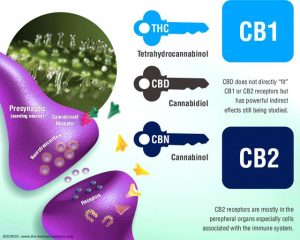
CBD is a type of cannabinoid, a chemical compound found in many plants, but mainly associated with cannabis.
Unlike tetrahydrocannabinol (THC), the primary cannabinoid found in cannabis, CBD does not produce a psychoactive effect, meaning users do not experience the feeling of being “high” associated with typical cannabis use.
Another benefit of CBD is that it is not addictive, with a very low toxicity level.
CBD offers therapeutic effect thanks to its anti-inflammatory, anticonvulsant, antipsychotic, and antioxidant profile.
It is increasingly becoming popular due to its different recreational and medical benefits.
Benefits
Undergoing research has lifted the stigma associated with CBD and paved the way for research and use in medical industry.
However, it is important to understand that different strains of cannabis produce different effects depending on the ratio of CBD and THC present in it.
Recent research has shown CBD promotes ‘wakefulness,’ therefore has been recommended for treatment of anxiety and depression.
Patients looking to treat these symptoms should look for a strain with higher percentage of CBD and low THC, for example, CD1 (a recent cannabis cup winner) or Charlotte’s Web which have ratios of 20:1 CBD:THC

Some of the potential benefits that CBD is being used for includes:
- Cancer– Study conducted by the National Institute of Health has shown that cannabinoids have anti-tumor properties and reduce inflammation. It is also helpful in reducing the painful side effects of chemotherapy.
- Depression – A study from the University of Rio de Janeiro showed that CBD has considerable potential in treating anxiety and depression along with treatment for its symptoms like insomnia and nausea.
- Diabetes – A study published in the American Journal of Medicine found that cannabis use lowered the fasting insulin levels and lowers incidence of diabetes.
How does it work?
Every human body has a receptor system known as the Endocannabinoid System (ECS), which is responsible for maintaining and protecting body’s healthy state.
The human body, through the ECS, produces its own cannabinoids which can influence inflammation, pain, and mood.
Cannabis, and cannabis products such as CBD isolates, stimulates the ECS by supplementing these cannabinoids to help the body maintain a healthy functional state.
Cannabinoids from cannabis may be thought of as a “key,” which acts on receptors along the ECS.
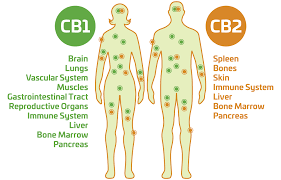
The two receptors in the ECS are the CB1 and CB2.
CB1 receptors are located along the central nervous system, while CB2 receptors are more often found on immune cells, for example in the gastrointestinal tract or the peripheral nervous system.
CBD has the potential to alter the emotional reaction to pain. It does not directly affect the pain centers of the brain, hence is not addictive like opioids.
How can you use CBD?
The easiest and most efficient way to use CBD is in the form of an oil. CBD oil can also be taken in form of pills.

CBD is also available in the form of topical creams or tinctures which can be less concentrated than oil.
Topicals can be directly applied to the skin, especially useful for combating symptoms related to inflammation. These have been highly recommended by people suffering from arthritic joint pain or muscle aches.
CBD oil can even be consumed by vaping or sprays.
Vaping allows the medicine to enter body quickly while avoiding the harmful side-effects of smoking. Concentrated tinctures can be used as a spray under the tongue.

A new type of cannabis product delivers both quick and long-lasting relief without the high is CBD patches. For delivering medication into the bloodstream, these transdermal patches are convenient, discreet, and effective.
How is CBD better than conventional painkillers?
Using CBD for pain relief offers many advantages over conventional medicines:
- The risk of overdose fatality is negligible
- No withdrawal symptoms
- Non-addictive
Side Effects?
While CBD is considered generally safe with only few mild side effects, like any drug it can sometimes come with side-effects.
These side-effects, however, have consistently been reported to be significantly less severe than those associated with traditional pharmaceutical medications.
Some of the reported possible side-effects include:
- Fatigue
- Diarrhea
- Drowsiness
- Loss of appetite
Some people are allergic to CBD oil and therefore application on a small patch of skin is recommended first.
References and further Reading
http://www.amjmed.com/article/S0002-9343(13)00200-3/abstract
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3202504/
https://www.ncbi.nlm.nih.gov/pubmed/24923339
Key Milestones in Medical Use of Cannabis: the Discovery of the Endocannabinoid System
Key Milestones in Medical Use of Cannabis: the Discovery of the Endocannabinoid System
- “By using a plant that has been around for thousands of years, we discovered a new physiological system of immense importance,” Raphael Mechoulam
For anybody interested in the increasing use of cannabis to treat medical conditions, it is worth recalling some of the scientific events underpinning this situation.
These events have not only helped to change our understanding of how cannabis exerts its effects in the human body, but also on our way of thinking about the potential uses of cannabis.


“I believe that small, regular doses of cannabis might act as a tonic to our most central physiological healing system.”
– Dustin Sulak
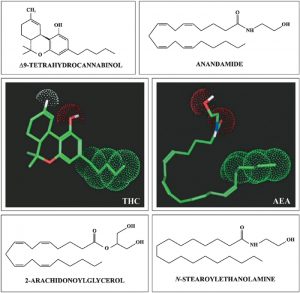
-
Endogenous arachidonate-based lipids, [anandamide (N-arachidonoylethanolamide, AEA) and 2-arachidonoylglycerol (2-AG)]; these are known as “endocannabinoids” and are physiological ligands for the cannabinoid receptors (ligands are substances that form complexes with biomolecules to serve a biological purpose).
-
The enzymes that synthesise and degrade the endocannabinoids, such as fatty acid amide hydrolase or monoacylglycerol lipase.
-
The cannabinoid receptors CB1 and CB2, two G protein-coupled receptors that are located in the central and peripheral nervous systems.
-
The neurons, neural pathways, and other cells where these molecules, enzymes, and one or both cannabinoid receptor types are all co-localised from the endocannabinoid system.
References and further Reading
1. Gaoni, Y. and Mechoulam, R. (1964). Isolation, structure and partial synthesis of an active constituent of Hashish. Journal of the American Chemical Society 86, 1646–1647.
2. Devane, W.A., Dysarz, F.A. 3rd, Johnson, M.R., Melvin, L.S. and Howlett, A.C. (1988). Determination and characterization of a cannabinoid receptor in rat brain. Molecular Pharmacology 34, 605–613.
3. Matsuda, L.A., Lolait, S.J., Brownstein, M.J., Young, A.C. and Bonner, T.I. (1990). Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 346, 561–564.
4. Munro, S., Thomas, K.L. and Abu-Shaar, M. (1993). Molecular characterization of a peripheral receptor for cannabinoids. Nature 365, 61–65.
5. Devane, W.A., Hanus, L., Breuer, A., Pertwee, R.G., Stevenson, L.A., Griffin, G., Gibson, D., Mandelbaum, A., Etinger, A. and Mechoulam, R. (1992). Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science, 258, 1946–1949.
(1995). Pertwee, R.G., Griffin, G., Bayewitch, M., Barg, J. and Vogel, Z.
6. Mechoulam, R., Ben-Shabat, S., Hanus, L., Ligumsky, M., Kaminski, N.E., Schatz, A.R., Gopher, A., Almog, S., Martin, B.R., Compton, D.R., Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochemical Pharmacology 50, 83–90.
Can CBD Alleviate the Symptoms of Epilepsy?
Can CBD Alleviate the Symptoms of Epilepsy?
There have been many examples in recent years of published scientific articles, which have been peer-reviewed, as well as news items in the more general press, which have indicated the broad range of medical conditions susceptible to treatment by cannabis and related compounds.
Much publicity has been gained in particular for reports claiming to demonstrate the beneficial effects of cannabis on childhood epilepsy, a most distressing disease. A widely read and reputable US Journal “Scientific American” has published an article describing and analyzing the current data on this subject, which has been gathered in the US state of Colorado. The overall but not surprising conclusion of the article is that more and better-managed trials are essential. Drug companies are already working on compounds related to cannabis that show the benefits without the associated cognitive problems, which are linked to the psychoactive compound THC. GW Pharmaceuticals has developed two marijuana-derived drugs, Epidiolex and Sativex, the latter already available as prescription items in some countries. Epidiolex, a purified form of cannabidiol [CBD], is intended to treat epileptic seizures and is being tested in an international clinical trial led by the University of California, San Francisco, Epilepsy Center. It has already been granted orphan drug status—a path to approval based on smaller clinical studies than normal—by the US Food and Drug Administration [FDA].
“…it affects approximately 50 million people with about 1% showing active symptoms.“
The reported case of Charlotte Figi, a child whose nearly constant seizures were dramatically curtailed with cannabidiol, has now led to more large scale trials being started using established protocols [placebos and double-binding] to provide an acceptable medical, as opposed to anecdotal, basis for the arising results. A brief explanation of the neurological condition known as epilepsy will provide a context for these developments.
Epilepsy is a chronic neurological disorder of the human brain and has been seen in almost all countries across the world with the best estimates indicating that it affects approximately 50 million people with about 1% showing active symptoms. According to the World Health Organisation [WHO] the disease is characterised by recurrent seizures, which are brief episodes of involuntary movement that may involve a part of the body (partial) or the entire body (generalised). Other symptoms, usually of short duration, occur including loss of awareness and sensation (including vision, hearing and taste) and the loss of other cognitive functions as well as mood changes.
Although more data are needed, animal studies and clinical experience suggest that marijuana or its active constituents may have a place in the treatment of partial epilepsy.
[In the study] we present the case of a 45-year-old man with cerebral palsy and epilepsy who showed marked improvement with the use of marijuana. This case supports other anecdotal data suggesting that marijuana use may be a beneficial adjunctive treatment in some patients with epilepsy.”
Seizures are a result of excessive electrical discharges in a group of brain cells with the site of the discharge varying from person to person. The frequency of seizure events can vary from one per annum up to several per day. There has been much debate as to what constitutes epilepsy in terms of a clinical diagnosis but there is now general agreement among neurologists that epilepsy is defined as having 2 or more unprovoked seizures [this distinction became necessary when it was recognised that single seizure events might occur only once in a lifetime].
Misunderstanding of epilepsy is widespread with some popular beliefs concluding that epilepsy is contagious. This is not the case! Two types have been defined: idiopathic epilepsy, with no identifiable cause and is thus unavoidable, affecting 6 out of 10 people with the disorder; secondary epilepsy or symptomatic epilepsy with well-identified causes, which in principle could be avoided. Such causes include brain damage from prenatal or perinatal injuries (e.g. a loss of oxygen or trauma during birth); congenital abnormalities or genetic conditions with associated brain malformations; a severe head injury; a stroke that restricts the amount of oxygen to the brain; an infection of the brain such as meningitis, encephalitis, neurocysticercosis; certain genetic syndromes; a brain tumour.
WHO claims that epilepsy can be treated easily and affordably with daily medication costs as low as US $5 per year. Recent studies in both low- and middle-income countries have shown that up to 70% of children and adults with epilepsy can be successfully treated (with success being defined as complete control of seizures) with anti-epileptic drugs (AEDs). In contrast to drugs which have a curative effect anti-epileptic drugs are intended to prevent seizures from occurring. Some medications are taken as a ‘course of treatment’ for a disease or to cure a condition (for example, taking a course of antibiotics for an infection). Anti-epileptic drugs (AEDs) are different; they are a preventative medication taken every day to try and stop seizures from happening. They appear to do this by reducing the excessive electrical activity in the brain that causes seizures but the details of the mechanism are not well understood. The development of more than 20 compounds with anti-epileptic properties represents a major advance for sufferers.
The current interest in cannabis as an anti-epileptic agent is in part the result of claims that it has successfully been used to treat young children in the USA suffering from “Dravet’s Syndrome”, a debilitating condition characterised by constant seizures, often as many as several hundred per week. Treatment with a cannabidiol extract has been shown to reduce, in some instances, the number of seizures to less than 10 per week! The parents of children with this syndrome have been very vocal in their demands for the drug to be made available as soon as possible. Indeed some families have moved to Colorado to access the drug. The opposition has focussed on the lack of solid clinical trial data and is further compounded by the federal Schedule 1 drug classification, which effectively has blocked broad scientific research on cannabis. However, in late 2015, the United States Drug Enforcement Administration (DEA) eased some regulatory requirements imposed by the Controlled Substances Act (CSA) for those who are conducting FDA-approved clinical trials on cannabidiol (CBD). These changes may well improve the overall research process regarding CBD’s possible medicinal value and encourage ongoing scientific studies with a broad range of medical issues being addressed. The future is seemingly bright if the science is sound and politicians are supportive.
CBD Declared a Medicine: The Medicines and Healthcare products Regulatory Agency’s (MHRA) initial foray into British “Cannaland”
CBD Declared a Medicine: The Medicines and Healthcare products Regulatory Agency’s (MHRA) initial foray into British “Cannaland”
The Medicines and Healthcare products Regulatory Agency (MHRA) is an executive agency of the United Kingdom’s Department of Health. It is responsible for ensuring that medicines and medical devices work and are safe, of the required quality and show efficacy.
Medicinal products are defined in Article 1 of the European Parliament and Council Directive 2001/83/EEC. This has been incorporated into the 2012 Human Medicines Regulations statutory instrument of UK law. One provision of this act is that medicinal products that are placed on the market must have marketing authorisations. One consequence of this act is that the sale, supply, advertising of medicinal products, which do not have such marketing authorisation, constitutes an offence. Medicinal products have been carefully defined so that they include substances which have properties that include treatment and prevention of disease in human beings, in addition to the restoration, correction or modification of physiological functions by exerting a pharmacological, immunological, or metabolic action.
“It would appear that food supplements can be classified separately from medicines and to date the MHRA has not made any determination in relation to their status“
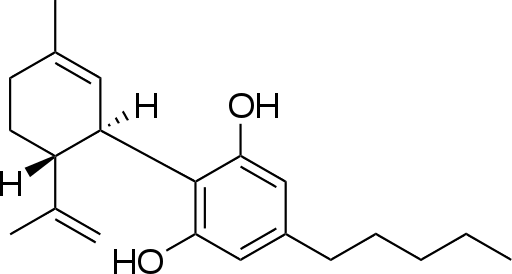
Furthermore, the Act also covers means of making a medical diagnosis. The MHRA has recently completed a review of Cannabidiol (The International Union of Pure and Applied Chemistry (IUPAC) nomenclature of this compound is: 2-[(1R,6R)-6-isopropenyl-3-methylcyclohex-2-en-1-yl]-5-pentylbenzene-1,3-diol) and has come to the opinion that it meets the second part of the definition of a medicinal product hence medicinal claims cannot be made for cannabidiol (CBD) in the absence of a marketing authorisation. Efficacy usually requires clinical trial evidence and in its absence products cannot be marketed. To this end, in early October this year the MHRA has been writing to 18 known vendors/distributors of CBD, which are believed to have made claims for medical effects arising from its ingestion by human beings. In essence the letter provides a brief summary of the legal status of products containing CBD in the UK. It is worthwhile examining the details of the subsequent statement made by the MHRA, via a spokesperson, published on their website.
“We have come to the opinion that products containing cannabidiol (CBD) used for medical purposes are a medicine. Medicinal products must have a product licence (marketing authorisation) before they can be legally sold, supplied or advertised in the UK, unless exempt. Licensed medicinal products have to meet safety, quality and efficacy standards to protect public health.”
“If you use CBD and if you have any questions, speak to your GP or other healthcare professional.”
“We have written to UK CBD stockists and manufacturers to inform them of our view. We can provide regulatory guidance to any company who may wish to apply for a licence.”
An initial reaction to this statement is to question its relevance to hemp products, such as hemp seed oil, that are not intended for therapeutic use. Indeed, at the time of writing no correspondence from MHRA on this matter has been received by hemp producers. The sale and distribution of CBD as a food or foodstuff appear to be governed by regulation no. 178/2002. The Food Supplements Directive 2002/46/EC in outline defines a food supplement as ‘something which supplements a normal diet and is a concentrated sources of nutrients or other substances with a nutritional effect’. CBD can be categorised as an “other substance” and is therefore subject to national laws which must comply with this directive and in particular cannot be labelled, presented, nor advertised as preventing, treating, or curing a human disease. The precise legal issues are complicated and will require a detailed assessment of the claims made about each product. In truth, the MHRA has emphasised the fact that it assesses each product on a case by case basis rather than the application of a blanket ban. It would appear that food supplements can be classified separately from medicines and to date the MHRA has not made any determination in relation to their status. Clearly, this is a situation that requires very careful consideration of all the relevant factors and one would hope that the MHRA would respond positively to representations made to it by producers of CBD intended for use solely as a food supplement (with no claims being made for medical effect). Other issues among others which merit more detailed scrutiny include: CBD’s therapeutic effect and its value; the right to medicines under European Union (EU) law; the free movement of goods within the EU; a reasonable period of time being made available to companies operating in this space so that they can make formal representations to the MHRA; a list of existing licenses granted for the treatment of specific conditions such as arthritis, multiple sclerosis, childhood epilepsy, various forms of cancer and in particular pain management related to such conditions; the origin and nature of the clinical evidence (animal/human) to be provided in support of any application for a licence; the financial cost of applying for a licence.
It is clear that there will be a variety of opportunities available to the industry to enter into a constructive dialogue with the MHRA. The fact that an increasing number of countries and states are envisaging or are enacting legislation around the world to cover the wider use of cannabis for medical purposes adds further weight to the case for detailed discussion with the MHRA, which could usefully include a formal consultation process with all relevant stakeholders. It is to be hoped that such a process can be engaged before the end of the year.
E-H.O.DESFORGES © 2016
The “Adult Use of Marijuana Act” a.k.a “Proposition 64”: how will the Californian electorate vote on November the 8th?
[fullwidth background_color="" background_image="" background_parallax="none" enable_mobile="no" parallax_speed="0.3" background_repeat="no-repeat" background_position="left top" video_url="" video_aspect_ratio="16:9" video_webm="" video_mp4="" video_ogv="" video_preview_image="" overlay_color="" overlay_opacity="0.5" video_mute="yes" video_loop="yes" fade="no" border_size="0px" border_color="" border_style="" padding_top="20" padding_bottom="20" padding_left="" padding_right="" hundred_percent="no" equal_height_columns="no" hide_on_mobile="no" menu_anchor="" class="" id=""][separator style_type="single" top_margin="30" bottom_margin="15" sep_color="#388e47" border_size="" icon="fa-envira" icon_circle="" icon_circle_color="#ffffff" width="" alignment="center" class="" id=""][fusion_text]
On November the 8th this year electors in 9 States of the US Federal Union will be voting on specific propositions for changes in State laws governing the production, possession, use, and distribution of marijuana.
This is to be viewed against the background of U.S federal law which effectively outlaws the growth, possession, and use of marijuana, which is regarded as being a controlled substance for the purposes of federal law. Infringements of the law are penalised.
Of the nine states voting the most significant is the State of California, which had originally considered Proposition 19, also known as the California Marijuana Initiative (CMI), on the November 7, 1972 California statewide ballot. This was the first attempt to legalize marijuana by ballot in the history of the United States. 44 years later California is submitting Proposition 64, a voter-led initiative, for decision by the electorate. The proposition which is a 62 page document with an enormous amount of detail (an experienced U.S lawyer claims to have taken 36 hours to have read every single provision of the proposal in order to fully understand both the principles of and the action of the Act). Essentially, the Act is proposing to legalise the public possession of a maximum amount of 1 ounce of marijuana and the private ownership for growth and cultivation purposes of 6 plants. One specific provision of the Act is that all retail sales will be subject to a 15% sales tax. The approval of this proposition will lead inevitably to a fundamental conflict between federal law and state law.
The fierce public debate on this proposition, prior to the November election, has centred on issues relating to control by the state of the production and sale of a substance, which apart from approved medical use, has been in the hands of criminals and in contrast to tobacco and alcohol has not been subject to regulation and taxation. Issues of individual liberty and civil rights have always complicated this debate as well as raising public morality issues. One immediate consequence of this measure being approved will be that the state will no longer be criminalising adults nor imprisoning children. The proposed reforms are estimated to be capable of saving the taxpayer $100 million annually in current costs and in contrast will raise approximately $1 billion in new tax revenues. Supporters of the proposition claim that the majority of these revenues will be allocated to teenage drug education and treatment, law enforcement with respect to driving under the influence, environmental protection from the harm arising from illegal cultivation, as well as providing funds to support local economic initiatives where communities have been adversely impacted by the prohibition of marijuana. Opponents contest this commitment.
[/fusion_text][one_fourth last="no" spacing="yes" center_content="no" hide_on_mobile="no" background_color="" background_image="" background_repeat="no-repeat" background_position="left top" hover_type="none" link="" border_position="all" border_size="0px" border_color="" border_style="" padding="" margin_top="" margin_bottom="" animation_type="" animation_direction="" animation_speed="0.1" animation_offset="" class="" id=""][separator style_type="single|dashed" top_margin="30" bottom_margin="30" sep_color="#388e47" border_size="" icon="" icon_circle="" icon_circle_color="" width="" alignment="center" class="" id=""][fusion_text]
"The proposed reforms are estimated to be capable of saving the taxpayer $100 million annually in current costs and in contrast will raise approximately $1 billion in new tax revenues..."
[/fusion_text][separator style_type="single|dashed" top_margin="30" bottom_margin="30" sep_color="#388e47" border_size="" icon="" icon_circle="" icon_circle_color="" width="" alignment="center" class="" id=""][/one_fourth][three_fourth last="yes" spacing="yes" center_content="no" hide_on_mobile="no" background_color="" background_image="" background_repeat="no-repeat" background_position="left top" hover_type="none" link="" border_position="all" border_size="0px" border_color="" border_style="" padding="" margin_top="" margin_bottom="" animation_type="" animation_direction="" animation_speed="0.1" animation_offset="" class="" id=""][fusion_text]
The origins of the Act are to be found in bi-partisan efforts to control and regulate the medical marijuana industry. Additionally, the Act incorporates best practices to be found in the various states as well as lessons from practical implementation of legislative changes elsewhere. More specifically, the recommendations of a commission on marijuana policy have been included in the Act. Strict anti-monopoly provisions and a system to protect small farmers can be seen in the Act so as to counter criticisms that the marijuana industry will be simply overrun by large corporations. One of the dangers of this regulatory approach is that marijuana will be treated as a most valuable cash cow to be milked, at both regional and local level, whenever deficits appear in other parts of the state budget. An excessive tax burden could well push buyers of marijuana back into the hands of the black market. A most undesirable outcome!
Concern has been expressed about the availability of marijuana to minors under the provisions of the new Act and especially to children. However, the sale of marijuana edibles as “candy” will be expressly prohibited by terms such as “appealing to children and capable of being confused with other candies” although stronger language might have been used. A more serious financial critique has been raised by various activist groups, which claim that the new Act is fundamentally based on a business model rather than using a public health framework such as underpins the production and sale of alcohol and tobacco. Allegations have been raised that large corporations have been channelling money in support of the proposition in the hope that post the election economic developments will allow domination of the market by these aforementioned corporations.
[/fusion_text][/three_fourth][testimonials design="classic" backgroundcolor="#388e47" textcolor="#e8e8e8" random="" class="" id=""][testimonial name="Margaret Dooley-Sammuli" avatar="image" image="http://medicalmarijuana.co.uk/wp-content/uploads/2016/09/Margaret-Dooley-Sammuli.jpg" image_border_radius="round" company="Drug Policy Director - California ACLU" link="http://www.cwcbexpo.com/speaker-lineup/margaret-dooley-sammuli/" target="_self"]"It is time to move from prohibition to regulation...establish a controlled and regulated market for adults, significantly reduce the harm done to young people under current marijuana laws, and generate substantial revenue for drug education and for the communities most devastated by the war on drugs." [/testimonial][/testimonials][one_full last="yes" spacing="yes" center_content="no" hide_on_mobile="no" background_color="" background_image="" background_repeat="no-repeat" background_position="left top" hover_type="none" link="" border_position="all" border_size="0px" border_color="" border_style="solid" padding="" margin_top="30" margin_bottom="" animation_type="0" animation_direction="down" animation_speed="0.1" animation_offset="" class="" id=""][fusion_text]
Clearly there is a clash between a market approach and one based on public health and the Act does not resolve this difficulty. It is worth noting that one of the more credible analyses has been advanced by the “Centre for Tobacco Control, Research and Education”, which is based at the University of California, San Francisco in a paper published in February 2016. The authors claim that the goal of any marijuana regulatory framework should be to treat marijuana regulation like tobacco regulation, allowing sale and use to be legal, while simultaneously creating an environment where falling numbers of people are interested in buying and using it. To minimize public health risks, it is claimed that marijuana regulations in California should be modeled on the California Tobacco Control Programme. One specific point raised is the apparent failure to consider the economic impact of marijuana legalization on increasing health costs in California. This presumes that the marijuana will have a similar impact on general health to that of tobacco. Experience in years to come will prove or deny the truth of this assertion.
Those opposed to the proposition for the legalization of marijuana in general base their concerns on a number of unrelated issues most of which appear to be potential rather than factual. One line of criticism suggests that cartels may buy up land and then monopolize the industry through violence and intimidation. Criminal efforts may depend on those with prior convictions of dealing in large amounts of controlled substances becoming licensed marijuana dealers. Another legal issue concerns driving under the influence of drugs and critics have pointed out that insufficient investment has been made in developing viable testing protocols and speculate as to the quantum and source of the necessary expenditure to enforce them. More significant will be the issue of the residence time of detectable residues in drivers and relating this to a prior consumption event. None of these are insurmountable obstacles!
A point of legal debate with respect to juveniles, those under the age of 21, will be the exact location of growing sites since there appears to be confusion as to whether indoor and outdoor facilities are equivalent under the Act ,with respect to the category of offence being committed.
How will this play out? The support for the proposition is broad-based with support from the California Academy of Preventative Medicine; the California Medical Association; sitting California Lt Gov. Gavin Newsom; bipartisan elected officials in the US Congress and an unprecedented coalition of environmental, business owners, small farmers, civil rights, public safety and social justice groups. A conservative electorate may still have sufficient power to refuse approval once again but the tide is turning and this will surely be the last occasion for them to hold back popular forces of liberty.
[/fusion_text][/one_full][section_separator divider_candy="top" icon="" icon_color="#388e47" bordersize="1px" bordercolor="" backgroundcolor="" class="" id=""][/fullwidth]