Research shows medical cannabis has potential to alleviate symptoms in autistic children
Research shows medical cannabis has potential to alleviate symptoms in autistic children
- Researchers for Tikun Olam found medical cannabis can treat Autism symptoms in children
- 30% of patients studied reported a significant improvement in quality of life
- 34.3% reduced use of other medications
- Majority of patients were treated with a 20:1 CBD:THC tincture
- Autism affects 2.3 million families in the UK
Treating autism spectral disorders (ASD), especially in children, has presented a notoriously difficult problem for the medical profession.
Up to now, evidence that medical cannabis could have the potential to be used in the treatment of ASD has largely been anecdotal.
Researchers for Tikun Olam, including legendary cannabis scientist Raphael Mechoulam, have published a study which found cannabis to have properties which help alleviate some of the symptoms of ASD in children.
The study, published in Scientific Reports, shows how cannabis offers a safe and effective option for patients, able to deduce symptoms such as seizures, ‘rage attacks’, restlessness, tics and depression in children.
Researchers analysed the treatment programmes of 188 ASD patients, aged 18 or under, who were being treated with medical cannabis between 2015 to 2017.
The majority of patients were treated with a cannabis oil containing 30% CBD and 1.5% THC.
For children who reported high sensitivity to previously used medications, the treatment started with oil containing 1:20 15% CBD and 0.75% THC.
Patients with severe sleep disturbances, or violent behaviour, an extra 3% THC was added following the initial treatment phase.
Finding the optimal ratio of cannabinoids varies from patient to patient, something the researchers were careful to account for.
Each patient’s dose was gradually increased, depending on the effect the cannabis was having on targeted symptoms. Parent’s determined their child’s individual treatment plan, accounting for the child’s tolerance to previous medication and cannabis.
Autism is different for every child, with each needing a different ratio of cannabinoids for their specific ASD. Finding the optimal dose could take up to two months.
The study found that the range of dosage between patients was wide.
Some patients needed only one drop three times a day, while others needed up to 20 drops three times a day of the same product.
Researchers contacted parents after one month for a follow-up on the progression of the treatments.
179 patients were assessed after six months, undergoing an additional assessment of the symptom intensity, side effects and quality of life.
A further 93 (60.0%) responded to a questionnaire.
This real, honest self-assessment by parents of children with ASD provides significant evidence that medical cannabis can help families of children with ASD.
Nearly all of the participants reported an improvement in their quality of life.
28 patients (30.1%) reported a significant improvement, 50 (53.7%) a moderate improvement, 6 patients (6.4%) a slight improvement, with 8 (8.6%) reporting no change in their condition.
Overall, good quality of life was reported by 31.3% of patients prior to treatment initiation while at 6 months, good quality of life was reported by 66.8%.
Positive mood was reported by the parents on 42% before treatment and 63.5% after 6 months of treatment,
The ability to dress and shower independently was significantly improved from 26.4% reported no difficulty in these activities prior to the treatment to 42.9% at six months.
Good sleep and good concentration were reported by 3.3% and 0.0% (respectively) before the treatment and on 24.7% and 14.0% during an active treatment.
Restlessness and rage attacks were improved in 72 patients (91.0%) and 66 (90.3%) respectively.
30% of patients studied reported a significant improvement in quality of life
ASD can be a heavily medicated illness. Of the 93 patients who responded to the follow-up questionnaire, 67 reported initial chronic use of medications
The most common concomitant chronic medications on the intake were antipsychotics (56.9%), antiepileptics (26.0%), hypnotics and sedatives (14.9%) and antidepressants (10.6%).
Over a third of patients were able to reduce their intake of other medications (23 patients, 34.3%), while 38 patent’s medication use remained the same (56.7%). Interestingly, six patients (8.9%) reported an increase in their drugs consumption.
While the results from the 2 year study are a positive step-forward in the future treatment of ASD in children, more research will be needed before any conclusions can be made on the efficacy of cannabis for ASD,
Senior study investigator Victor Novack, concluded:
“While this study suggests that cannabis treatment is safe and can improve ASD symptoms and improve ASD patients’ quality of life, we believe that double-blind placebo-controlled trials are crucial for a better understanding of the cannabis effect on ASD patients.”
Clearly, medical cannabis has a great potential to be used in future treatments on ASD.
With around 700,000 people on the autism spectrum in the UK, should the British medical profession be looking at the possibility of including ASD as a qualifying condition for legal access?
Research finds smoking cannabis can cause remission in Crohn’s patients
Research finds smoking cannabis can cause remission in Crohn’s patients
- Israeli scientists have proof cannabis can help Crohn’s patients enter remission
- 45% of patients given THC entered full remission in 8 weeks
- Around 115,000 in the UK are affected by Crohn’s
With recent surveys suggesting that new cases of Crohn’s disease are being more diagnosed more often, could medical cannabis offer a better and safer treatment than currently used, pharmaceutical medications?
According to research from 2013, cannabinoids could hold the answer.
Israeli scientists at Tel Aviv University conducted the worlds first placebo-controlled trial on cannabis and Crohn’s Disease, finding that 45% of Crohn’s patients they tested were able to enter remission using cannabinoid medication.
Researchers examined 21 patients with drug-resistant Cronh’s over a 8 week period to see if smoking cannabis containing THC could help provide relief where pharmaceutical medicine failed.
Half the group were provided cannabis cigarettes containing 115mg of THC, while the control group were provided with a placebo containing cannabis flowers from which the THC had been extracted.
Complete remission was achieved by 5 of 11 subjects in the cannabis group (45%), and only 1 of 10 in the placebo group (10%).
The authors concluded:
“Although the primary end point of the study (induction of remission) was not achieved, a short course (8 weeks) of THC-rich cannabis produced significant clinical, steroid-free benefits to 10 of 11 patients with active Crohn’s disease, compared with placebo, without side effects.
“Further studies, with larger patient groups and a nonsmoking mode of intake, are warranted.”
“…a short course of THC-rich cannabis produced significant clinical, steroid-free benefits to 10 of 11 patients with active Crohn’s disease, compared with placebo, without side effects.“
While the researchers were not able to achieve full remission in all of their patients, they did find that THC can offer effective and safe treatment to Crohn’s sufferers who are unable to be treated with traditional medications.
Those patients who were able to enter remission with THC were able to do so without side-effects, which can often be as debilitating as the illness itself.
Crohn’s is becoming a more common disease in the UK, with around 115,000 patients diagnosed with the chronic illness.
While some patients are able to find relief in pharmaceutical medications, studies like this one show that medical cannabis must be an option available for Crohn’s patients, especially those who have tried and failed to get results with traditional treatments.
References and further Reading
Research finds CBD has potential to be used in skin cancer treatments
Research finds CBD has potential to be used in skin cancer treatments
- New research suggests CBD could be used to help treat skin cancer
- Mice tested with CBD lived longer than those untreated, and had a greater quality of life than those treated with traditional cancer medication
- Skin cancer is the 5th most common cancer in the UK
A new study has found that Cannabidiol (CBD) has the potential to be used in future treatments of skin cancer.
Scientists at the Augusta University Medical Center researched the potential effectiveness of CBD in the treatment of skin cancer, specifically melanoma.
Using lab mice diagnosed with melanoma, split into three sections: those treated with CBD, those treated with a common anti-cancer drug, Cisplatin, and a control group.
The mice treated with Cisplatin received twice-weekly injections of a 5mg Cisplatin solution. CBD treated Mice were provided with a 5mg CBD oil. The control group was given no treatment.
The mice treated with Cisplatin lived the longest, while those who received no treatments quickly died.
The CBD-treated mice treated did live significantly longer than the control group, exhibiting a greatly improved quality of life (researchers determined this by studying the mice’s behaviour) than the Cisplatin group.
While the Cisplatin-treated mice did live longer than those treated with CBD, they demonstrated a heavy reduction in movement and an increase in aggressive tendencies.
The authors concluded:
“We demonstrate a potential beneficial therapeutic effect of cannabinoids, which could influence the course of melanoma in a murine model.
“Increased survival and less tumorgenicity are novel findings that should guide research to better understand the mechanisms by which cannabinoids could be utilized as adjunctive treatment of cancer, specifically melanoma.
“Further studies are necessary to evaluate this potentially new and novel treatment of malignant melanoma.”
“We demonstrate a potential beneficial therapeutic effect of cannabinoids, which could influence the course of melanoma in a murine model.“
From the evidence presented by the study, we can see that CBD does have the potential to be used in future skin cancer treatments.
While rats treated with the traditional cancer treatment lived longer, their quality of life was much lower than those treated with CBD. However, those treated with CBD lived longer and had a better quality of life than those untreated.
Further research will be needed to provide more conclusive evidence on the efficacy of CBD treatment in skin cancers in humans.
However, there is already research which has found that CBD is effective at treating cancers in humans.
There is even research that cannabinoids can work in conjunction with traditional chemotherapy treatments.
Looking at how mice treated with CBD had a better quality of life, and how mice treated with Cisplatin lived the longest, theoretically, treatments which combine the two could potentially help patients live longer and have a higher quality of life.
Skin cancer is the 5th most common cancer in the UK, accounting for 4% of all new cancer cases. There are around 2,400 melanoma skin cancer deaths in the UK every year, that’s more than 6 every day.
Melanoma is an easily treatable cancer if caught early. However, there is currently no effective treatment if it is diagnosed in its advanced stages. Unfortunately, around 1 in 10 melanoma skin cancer cases are diagnosed at a late stage in England.
While more research is needed, this new study offers hope to thousands of cancer patients in the UK. Including cannabinoids in cancer treatments offers the potential to greatly improve end of life care while potentially reducing the chance of fatality.
References and further Reading
We Need You! Have your views on cannabis heard by British Government
We Need You! Have your views on cannabis heard by British Government
Parliament’s Health and Social Care Committee is requesting the public to submit written applications to help inform their debate on the future of medical cannabis policies.
This is your opportunity as a patient to have your voice heard by those who are responsible for drug policy in the UK. This is your chance to get your personal experience with medical cannabis as a patient across to lawmakers.
You may even be asked to appear in person before the Committee to give a personal testimony in defence of legalising cannabis.
While you can submit the application anonymously, we at MMJ strongly advise that you include as much personal information as possible as this gives the movement more legitimacy: it puts a face to the countless patients who are crying out for legal access to medical cannabis.

Essentially, this application is the perfect opportunity to tell those who will decide whether or not you will be allowed to have legal medical cannabis why it is so important that you get this access.
To make the process easier, here are some guidelines to help you with the submission.
Remember: this is your opportunity to tell your story.
How to make a submission:
You can make your submission online.
- You will be asked on this page to fill in some personal details about yourself.
- You will be asked if you are submitting as an individual or an organisation: select ‘Individual’
- You must include your name, surname, email address and telephone number.
- It is optional to include your title, job title, and home address.
- You must upload your submission itself as a Microsoft Word Document (.doc). A guide for writing this submission can be found below

Submission Guide
Title: ‘Written evidence by [your name]’
1. Introduction (1-2 sentences):
- State who you are, and why you wish to submit to the consultation
- Are you a patient or a family member of a patient using medicinal cannabis?
2. What does your experience tell us about the efficacy of medicinal cannabis? (150-300 words)
- How did you find managing your condition before medicinal cannabis?
- How has medicinal cannabis changed this?
- What would your life be like without medicinal cannabis?
3. Do you find the current procedures for prescribing medicinal cannabis in the UK suitable? (150-300 words)
- Can you obtain medicinal cannabis for your condition in the UK?
- Can you obtain medicinal cannabis conveniently?
- Have you experienced difficulties in obtaining medicinal cannabis in the UK and if so, what were they?
- What needs to change to make your life better?
4. How knowledgeable is the medical profession when you seek advice regarding the medicinal cannabis products available for your condition? (150-300 words)
- What has your experience been when seeking prescriptions for medicinal cannabis?
- Does your doctor/consultant have the expertise in your opinion to confidentially prescribe medicinal cannabis?
- Have you experienced any negative feedback when seeking information or prescriptions for medicinal cannabis?
5. Have you been prescribed medicinal cannabis in another country? If so, how did your experience compare to your experience in the UK? (100-200 words)
- In which country were you prescribed cannabis?
- How was the process different from the process in the UK?
6. How has recent media attention around medicinal cannabis affected you? (100-200 words)
- Do you think people in the UK are aware of how medicinal cannabis can help in the treatment of conditions such as yours?
- Do you feel there is a stigma attached to medicinal cannabis in the UK?
Please note:
- This is a suggested guide only. You may choose to phrase your experience in your own way.
- It is strongly encouraged to use spell check and refrain from using slang or abbreviations. Take your time and be thoughtful.
World Health Organisation (WHO) recommends world governments reclassify cannabis
World Health Organisation (WHO) recommends world governments reclassify cannabis
- Experts at the United Nations will recommend cannabis and THC are rescheduled under international drug treaties
- Cannabis and THC are currently Schedule IV, the most restricted schedule
- WHO recommends cannabis is moved to Schedule 1, the lowest schedule
- The UN body also intends to remind governments that CBD is “not under international control
Global health experts at the United Nations are recommending that marijuana and its key components be formally rescheduled under international drug treaties.
The World Health Organisation (WHO) is calling for whole-plant cannabis to be moved from Schedule IV, the most restrictive category of a 1961 drug convention.
The body also expressed it desire for THC to be removed from another drug treaty, signed in 1971, instead placing the psychoactive cannabinoid alongside cannabis in Schedule I.
Schedule IV is exclusively reserved for substances which have limited, or no, medial benefits.
This scheduling is separate from British schedules. Cannabis was moved from Schedule I (the highest in Britain) to Schedule II in November 2018.
In the yet-unreleased report, WHO also made declarations that CBD, and CBD-based products containing less than 0.2% THC, are “not under international control.” CBD, unlike cannabis and THC, is not scheduled under WHO regulations.
The body will make recommendations that cannabis tinctures and other products containing extracted cannabinoids should be removed from Schedule I, with compounded pharmaceutical preparations containing THC would be placed in Schedule III of the 1961 convention.
Legally, the recommendations would not allow countries to legalise cannabis, but the political impact they could have are potentially enormous, especially for patients waiting for medical cannabis prescriptions.
Essentially, the recommendations would constitute a formal recognition that government’s across the world had been wrong on cannabis for the entirety of the global “War on Drugs.”
WHO’s newly adopted stand on cannabis coincides with numerous countries legalising cannabis for both medicinal and recreational purposes.
Plausibly, a change in policy direction at the UN could inspire individual nations to have a look into their own policies on cannabis; although, legalisation for recreational use would still violate other global conventions. But, as we have seen with Canada, those conventions are not necessarily binding.
The new proposals could be discussed as early as March before the UN’s Commission on Narcotic Drugs, where 53 member nations will have the opportunity to vote on accepting or rejecting them.
Russia and China have both historically blocked drug policy reforms, and are expected to oppose the change in cannabis’s classification.
On the other hand, nations like Uruguay and Canada and Uruguay, both of whom have legalised recreational cannabis, contravening current treaties in the process, are obviously likely to back the reform, as are a number of European and South American nations who now allow medical cannabis.
It is unknown how a Conservative-led Britain will vote. Historically, Britain has backed the US in most UN votes.
While the US traditionally pressures other nations to block cannabis reform, the fact that over half of the States in America have legalised access to cannabis either recreationally or medicinally, may make this stance untenable.
Under Trump, hemp was removed from the banned substance list, so there may be hope that the US may vote for the new proposals.
The WHO’s new cannabis rescheduling recommendations come in the form of a letter, dated January 24, from the Tedros Adhanom Ghebreyesus, the body’s director general, to UN Secretary-General Antonio Guterres.
Guterres was Prime Minister of Portugal when the country famously decriminalised all drug possession.
Regardless of WHO recommendations or policies, as shown by Canada and Uruguay, individual countries are responsible for their own drug policies.
Given the continued policy of ignoring scientific evidence by the British Government regarding cannabis, it is unlikely a Conservative-led Government would consider relaxing their approach on cannabis.
Research finds patients build a tolerance to CBD-isolated medicines
Research finds patients build a tolerance to CBD-isolated medicines
- Research has shown patients build tolerances to CBD-isolated medicines
- Evidence suggests THC could potentially reduce resistance
- Using different strains may also help reduce CBD tolerance levels
Medical cannabis was partially legalised in the UK in late 2018., but few patients have managed to obtain legal prescriptions.
Among those lucky few who do have legal prescriptions, many have been prescribed Epidiolex; a CBD-isolated medical cannabis tincture.
While the British Government is withholding full-extract cannabis oil, containing THC, patients left with CBD-only medications are suffering.
That is not to say CBD cannot work for everyone. Patients across the UK are finding great success with legal, non-prescribed CBD products, to the point where over 300,000 British citizens are now regularly purchasing CBD products.

However, for patients with severe epilepsy, CBD-only medications may not be good enough to help maximise a reduction in the severity and frequency of seizures.
Researchers in Israel published a study last year which investigated CBD tolerance levels in epileptic patients, both adult and child.
Ninety-two patients with treatment-resistant epilepsy were treated with a 20:1 CBD-THC cannabis oil extract for an average of 19.8 months.
Tolerance was defined as either the necessity to increase dose in 30% or more following reduction of efficacy, or response reduction of more than 30%.
The researchers found that a tolerance was seen in 30 (32.6%) of the patients, with the mean time till appearance of tolerance being 7.3 months.
Of those 30 patients, 17 (58%) showed > 50% reduction in mean monthly seizure frequency.
Researchers tried to combat the tolerance these patients were experiencing by increasing the CBD dose.
Only 12 patients were ale to see a reverse in the increase in tolerance with a increase in CBD dosage. For 15 patients, this had no effect.
The authors of the study concluded:
“Our findings suggest that Cannabidiol tolerance exists, and it limits the efficacy of this anti-seizure treatment in the long-term clinical management of epilepsy in the paediatric and adults population.”
Murray Gray, one of the only Scottish children to be prescribed Epidiolex, is one of these patients.
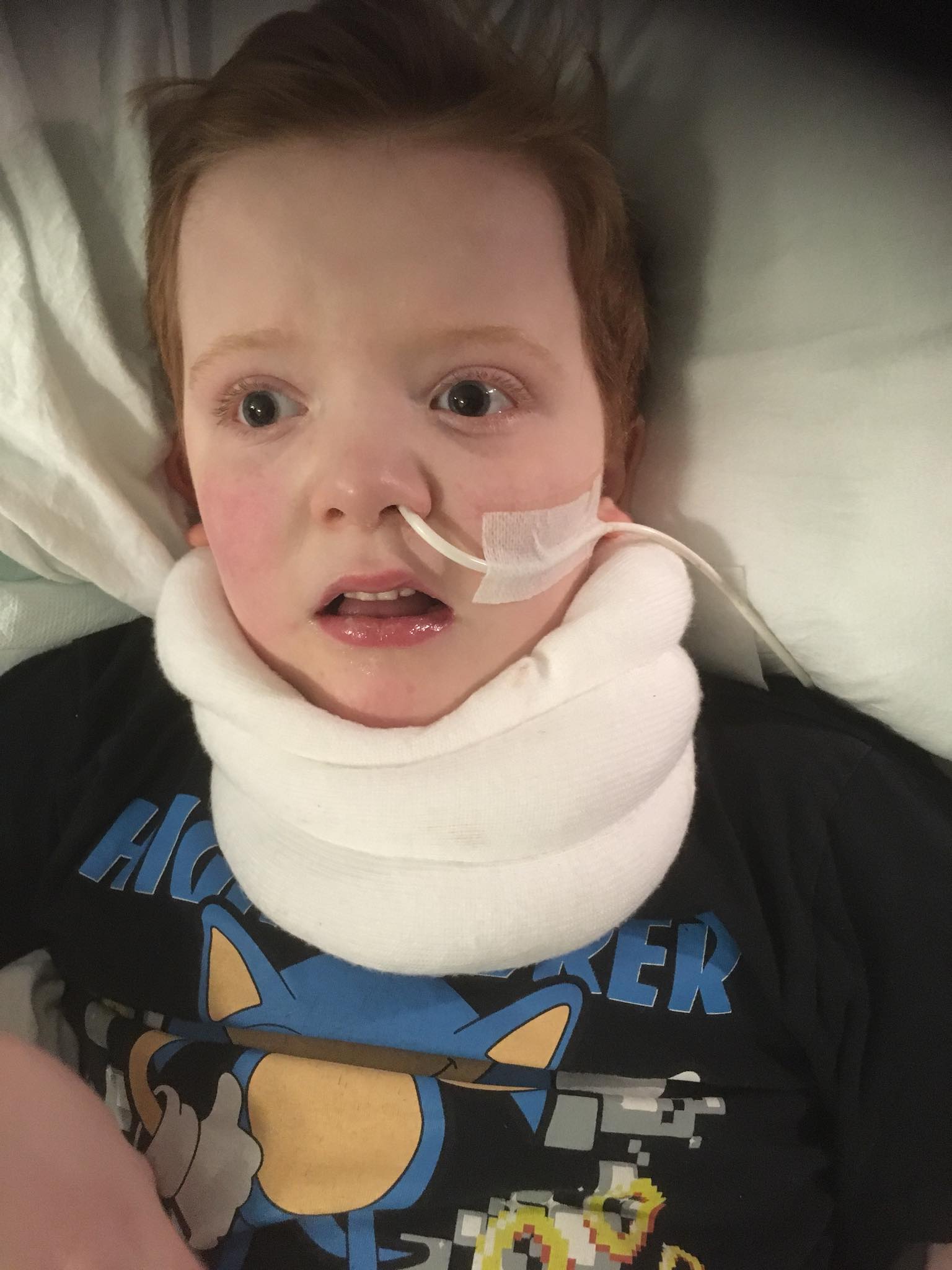
Murray has been prescribed Epidiolex since 2018, but its effectiveness is waning
Murray has been prescribed Epidiolex since August 2018. Within just two week, there was a great improvement in his condition.
Karen, Murray’s mother, explained:
“Murray’s daytime seizures vanished.
“However, he was still having nighttime tonic clonics [seizures], so he is still at risk of SUDEP (Sudden Unexpected Death in Epilepsy).
“His tonic clonics [seizures] also reduced in length.”

Could THC help the brave young boy recover?
Karen discussed with us how despite seeing an initial improvement with CBD-only products, their effectiveness wore off over time:
“Anytime Murray is ill, even just a little cold, Epidiolex stops working.
“We are back to tonics during the day, absences, myoclonics and drop seizures.
“After 5 months, Epidiolex no longer helps him.”
“I know of children who have benefited greatly from taking Bedrocan. Their seizures were either greatly reduced or stopped entirely!”
– Karen Gray, Mother and Medical Cannabis Warrior
Research suggests that the lack of THC, and other cannabinoids, in products like Epidiolex may explain their loss in effectiveness over time.
THC may be able to help reduce CBD-tolerance due to the way the two cannabinoids work on the Endocannabinoid System (ECS).
The ECS has 2 receptors: CB1 and CB2.
CB1 receptors are most commonly located within the brain, in the nervous system, and on the spinal cord and nerves, i.e., the areas which trigger seizures.
CBD only stimulates CB1 receptors. THC, on the other hand, actually activates CB1 receptors.
Essentially, THC’s interaction with CB1 receptors is superior to CBD’s.
THC triggers a full biochemical reaction in CB1 receptors, whereas CBD only lightly interacts with them, sending far tweaking signals from the receptor.
A study conducted by British researchers proposes that a 1:1 CBD:THC (equal parts CBD-THC), may help prevent a build up in tolerance to CBD. (https://link.springer.com/article/10.1007/s00415-014-7502-9)
The researchers used Sativex (another GW Pharmaceutical product) for their investigation. Each 100 µl spray of Sativex delivers 2.5 mg CBD and 2.7 mg THC.
380 patients with neuropathic pain, caused by diabetes or allodynia, took Sativex over a 38-week period.
Researchers found that not a single patient developed a tolerance to the spray.
However, the study only looked into CBD tolerance in relation to neuropathic pain, opposed to seizure prevention/reduction which was investigated in the Israeli study.
For children like Murray, 1:1 tinctures may be the best medical course to take.
“I want Murray to be prescribed Bedrocan’s products,” Karren told us.
“I know of children who have benefited greatly from taking Bedrocan. Their seizures were either greatly reduced or stopped entirely!”
While medical cannabis containing THC may be legal on paper, in reality, families like the Murray’s are finding it impossible to get a legal prescription for products containing both CBD and THC.
“The schedule change has made no different,” Karren explained.
“For children the BPNA are at fault.
“Their guidelines are wrong, and they need removing or updated to allow clinicians to prescribe THC to children.
“It’s the BPNA/pharmaceutical mafia that are blocking schedule 2.
“The only concern I previously had about THC came from listening to doctors telling me it will damage Murray’s brain damage. They are wrong. The BPNA are wrong.”
For children like Murray, a CBD tolerance can be life-threatening.
With more research emerging, providing clinical evidence that both CBD and THC are needed for the most effective treatment, how much longer must desperate patients wait for full spectrum prescriptions?
Karren has launched a petition for a change in the new medical cannabis guidelines provided by the British Government, to allow children to be more easily prescribed THC.
You can help her reach her goal here: https://you.38degrees.org.uk/petitions/medicinal-cannabis-oil-guidelines
40% of American patients ditch other pharmaceutical prescription drugs for medical cannabis
40% of American patients ditch other pharmaceutical prescription drugs for medical cannabis
- Over 2.1 million Americans have legal medical cannabis prescriptions
- A new University of Michigan study found that the 44% of respondent quit another drug altogether for cannabis
- 38% reduced the amount of a different drug they were taking
- Nearly one-third of medical cannabis patients don’t tell their doctor of their cannabis use
Yet another study has found significant evidence that cannabis can be used as an “exit drug” for patients addicted to prescription medications.
The study found that 44% of patients who use medical cannabis are able to quit taking at least one pharmaceutical prescription after receiving their medical marijuana prescription.
Over half of American states now have legal access to medical cannabis, with medical marijuana being legal in 34 States.
In America, cannabis has been approved to treat conditions such as pain management, nausea related to chemotherapy and even mental illnesses, such as depression.
For many conditions, however, doctors intend cannabis to be used a complement to other medications, not a replacement.
While doctors may intend for cannabis to only be a replacement, its superior effectiveness over pharmaceuticals is leading many patients to ditch their other prescriptions altogether.
The University of Michigan found in a survey that patients are often ditching their ‘primary’ prescriptions after they have been prescribed medical cannabis, with many refraining from telling their doctors about their choice.
It’s estimated that 2.1 million Americans now have official medical cannabis prescriptions (although the unofficial number may be far higher, with patients self-treating in legal States, or illegally self-treating in States where medical marijuana remains illegal).
Patients using medical cannabis are not always acquiring their prescription from their primary care provider. According to the study, nearly a third of patients are hiding their use from their primary care doctors entirely.
One reason for this may be that medical cannabis users are concerned that they may receive negative judgement from their doctors for their use of cannabis over opioid-based pharmaceuticals.
Out of 450 administered surveys, 392 respondents provided usable responses, with the majority answering that they had used marijuana recreationally in the past, suggesting a shift away from the Reefer Madness era which consumed the US from the 1950s.
While there has been a shift away from an overall negative perception of cannabis, a negative stigma remains on the Federal level.
Dr Daniel Kruger, the lead author of the study and a public health professor at the University of Michigan explained:
“Public health is still operating in the era of prohibition’ by leaving marijuana a Schedule 1 drug.
“It still treats cannabis use like ‘an abstinence-based program, like sex, and we know that doesn’t work.
“It’s not wild to say “here’s this plant that people have used medicinally for 5,000 years” … why are we not taking it seriously?’
Patient trust in cannabis is far higher than in pharmaceutical prescriptions.
“It’s not wild to say “here’s this plant that people have used medicinally for 5,000 years” … why are we not taking it seriously?“
– Dr Daniel Kruger, the lead author of the study
Every single respondent to the survey showed a preference for medical cannabis over pharmaceuticals, especially opioid-based ones, mainly due to its lack of negative side-effects and its non-toxic status (it’s impossible to overdose on cannabis).
According to the survey, 38% of medical cannabis patients were able to cut back on their use of another prescription drug after being prescribed medical marijuana, with 42% completely quitting their pharmaceuticals.
America is currently undergoing a severe Opioid Crisis.
Medical cannabis may be the only answer which can help end the suffering of millions addicted to dangerous, life-threatening opioid-based medications.
For example, in Colorado, where cannabis is legally available both medicinally and recreationally, opioid deaths have decreased by 6%.
According to Dr Kruger:
“People are not only self-medicating but they’re self un-medicating.”
Patient self-determination, i.e. self-medicating with cannabis without the guidance or knowledge of a doctor, could pose ‘serious dangers’ according to Dr Kruger:
“My concern is that we’re back to the mid-19th Century with people running around selling “cure-alls,” these tinctures that often have high potency.
“But, we don’t have the evidence-based science that is required for a standard pharmaceutical to see what this is effective at treating.”
While cannabis was moved to a Schedule 2 status in the UK last November, the herbal plant remains a Schedule 1 drug in the US, meaning there is a limit on what research scientists can legally conduct, with funding for cannabis research often being impossible.
Dr Kruger also discussed the limits on research, and his concerns over a lack of standardisation for which cannabinoids, and their ratios, in cannabinoid-based medications:
“It’s so illegal at the federal level that basically researchers still have one hand tied behind their backs and a giant boxing glove on the other.
“It’s like if a physician handed you a bag of pills of all different colours, shapes with maybe some numbers on them and they said ‘here, take them until you feel better.’
“That’s effectively the state we’re in.”
The University of Michigan’s study limited its scope for conditions which can be treated with both cannabis and traditional, pharmaceutical medications, in order to create a genuine comparison.
It’s worth noting, however, that some patients incorrectly believe that cannabis can treat conditions like cancer itself – not just side effects of chemotherapy, like nausea.
The study also found that the majority of respondents viewed medical cannabis as a more cost-effective treatment than its pharmaceutical counterpart.
Price comparison, however, was not the main reason patients were ditching their pharmaceuticals.
Respondents preferred cannabis due to other advantages, such as its minimal side-effects.
Dr Kruger believes that this new evidence should be used to push forward with legalising cannabis for medical consumption on a Federal level, in order to maximise safety for patients who are inevitably going to use medical cannabis:
“The science of the practice has not kept pace with the very rapid legal changes. All policy should be driven by science.
“There’s a lack of integration of these systems’ of the medical cannabis industry, the law, clinicians and public health policy.
“And integration of these systems is what we’re pushing for.”
Scottish Child One of Ten Wins Victory to be prescribed Medical Cannabis Oil
Scottish Child One of Ten Wins Victory to be prescribed Medical Cannabis Oil
- Scottish child, Cole Thomson, is set to be one of 10 children to receive a prescription for Epidolex
- Cole suffers drug-resistant epilepsy
- Up to 7000 children in Scotland have been diagnosed with epilepsy
The East Kilbride News revealed yesterday that Cole Thomson, 6, will be one of only 10 children in Scotland to be granted legal access to potentially life-saving medical cannabis.
Cole, from East Kilbride, is set to be one of only ten children in Scotland the Government has permitted to be prescribed cannabidiol (CBD) medication.
The victory follows a hard-fought campaign by his family and supporters to secure access to cannabis-based medication for Cole’s intractable epilepsy, with the news being confirmed yesterday that Cole will be prescribed CBD-based medication, Epidiolex.

Cole’s condition had been rapidly deteriorated rapidly over the last year, with the brave child suffering up to 10 life-threatening seizures a day, leaving the boy paralysed and unable to speak.
His family hope that the CBD could help reduce the number and severity of seizures Cole experiences, potentially preventing permanent damage to the young boy’s brain.
Lisa Quarrell, Cole’s mother, spoke to The East Killbride News to express her delight with her son’s new prescription:
“I have a mixture of emotions to be finally told Cole will have a prescription for Epidiolex from Glasgow – I’m proud, happy, relieved, angry and guilty.
“I’m so happy for Cole to finally be able to access medical cannabis in the UK and especially in Scotland.
“However, the journey to get here has been tough and exhausting which is completely unnecessary and unfair.
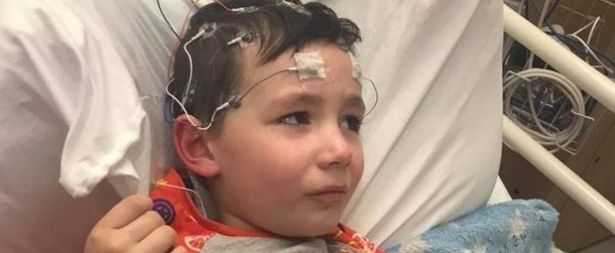
“There are still hundreds of children and young adults – some are part of our Parents of Hope group – who are still waiting and have been for years.
“Some with children who stop breathing and turn blue every night due to seizures, who are too drained to fight this fight publicly.
“My fight started about Cole and will always be about him. However, our journey brought us to where we are today and what we now know cannot be forgotten.”
“I’m so happy for Cole to finally be able to access medical cannabis in the UK and especially in Scotland. However, the journey to get here has been tough and exhausting which is completely unnecessary and unfair.“
– Lisa Quarrell, mother of Cannabis Warrior, Cole
While around 7,000 children in Scotland have been diagnosed with epilepsy, only 10 Scottish children are being allowed a prescription for medical cannabis.
Five children will receive cannabis oil at Edinburgh Sick Children’s Hospital, with a further five receiving it from Glasgow Children’s Hospital.
Despite an apparent change in the law in November 2018, which permits medical professionals to prescribe cannabinoid-based medication, children and adults across Scotland and the rest of the UK are being denied legal access due to overly restrictive guidelines.
Cole was initially refused treatment because he suffers from focal epilepsy and the brain defect cortical dysplasia.
His mother, Lisa, fought a hard campaign to raise awareness of Cole’s situation amongst policy-makers and politicians, while also raising thousands for other families in similar situations.
Lisa helped launch Parents of Hope campaign last year, which aims to reform the current laws and procedures for acquiring medical cannabis licenses.

Even with the change in the law, Lisa and her desperate family were forced to become “Cannabis Refugees,” trying to source whole plant cannabis treatment (which includes THC) from Spain.
Scottish MP, Monica Lennon, raised Cole’s case in Scottish Parliament last Thursday, bringing their story to Nicola Sturgeon’s attention.
He was offered medical cannabis treatment the next day.
Lisa also expressed a sadness that other children in Cole’s situation won’t be granted the same access he is:
“Cole is one of only five children in Glasgow picked out a hat, and 10 from the whole of Scotland to get Epidiolex until more is available in May.

“I have no doubt this is because I have been shouting about our pain and struggles. Now, after being told no for nearly a year, we finally get it.
“I will get Epidiolex in a few weeks and I pray this works for Cole because it’s all the UK have to offer.
“If this doesn’t work there’s nowhere to go here as my friend Karen Gray is discovering as she begs for her son Murray to be given a medication called Bedrolite which has already proved successful and life-changing and is already prescribed to children in England under private prescriptions.
“I want to continue to fight for all the children waiting and also for there to be a much clearer route for exhausted parents and for us to have options – not to feel pressure to accept the one thing on offer.
“Also, I need to know there’s a plan B if Epidiolex doesn’t work for Cole.”
While being given a prescription for CBD-based Epidolex it is certainly a positive step forward for Cole and his family, Karen Gary, mother of Murray, who also suffers intractable epilepsy and already has a prescription for Epidolex, has found the CBD-only medication has already lost its effectiveness.
The Edinburgh mum explained:
“When I started campaigning and found out the UK is the biggest provider of cannabis worldwide, I was outraged.
“Murray has been in hospital since last December.
“Two weeks after he started using Epidiolex we went home and he went back to school – his seizures gone.
“It’s crazy because there’s so many parents giving it to their kids illegally and just keeping quiet about it because it’s helping them and they’re so scared of getting arrested or a visit from social services but it’s helping their children lead normal lives – that’s wrong.
“They should be getting help by their doctors.”
Lisa thanked everyone involved in Cole’s campaign especially Monique McAdams of East Kilbride Community Trust, MP Dr Lisa Cameron, Monica Lennon MSP and everyone who fundraised for and donated to Parents of Hope.
A spokesman for NHS Greater Glasgow And Clyde said Epidiolex was “not yet licensed in the UK,” which means the administration of the drug to any patient is subject to a clinical assessment.
British mother fears severely epileptic son will die after hospital blocks its own consultant prescribing medicinal cannabis
British mother fears severely epileptic son will die after hospital blocks its own consultant prescribing medicinal cannabis
- Ben Griffiths, 9, suffers drug-resistant epilepsy and cerebral palsy, experiencing up to 300 seizures a day
- In October he was prescribed Epidiolex, but Alder Hey Children’s Hospital is preventing it being administered
- His mother believes the decision is putting her son at serious risk of permanent disability
A British mother has condemned Alder Hey Children’s Hospital for denying her severely epileptic son medicinal cannabis he could potentially die without.
Ben Griffiths, 9, from Lancashire, was born with cerebral palsy. At just six-months-old he was diagnosed with severe epilepsy, which has since been found to be drug-resistant.
The brave child’s condition is so severe it has caused multiple serious injuries, putting Ben in hospital twice last year with a suspected fractured skull.
Surgery has been put forward as an option, but carries the potential to further disable the child.
Great Ormond Street, where Ben was being treated before 2018, told his parents Joanne and Paul, that they could so no more for their child.
Joanne and Paul decided against surgery because it brings a high risk that he will be physically disabled.
Speaking to The I, Joanne, his mother, said:
“He already has a weakened right side from his cerebal palsy but he can walk – we don’t want to cause him any further brain damage.”

Ben was prescribed Epidolex last October after his seizures worsened. Epidolex contains less than 0.1% THC.
According to The Guardian, more than 50 children in the UK have already been treated with Epidolex on a compassionate access programme, which allows a patient with an immediately life-threatening condition or serious disease or condition to gain access to an investigational medical product (i.e. medical cannabis).
Despite Ben’s neurologist giving his backing for the use of medication containing more than 0.1% THC, the move has been blocked by Alder Hey following a panel meeting.[/fusion_text][fusion_text]In a tearful interview with The I, Joanne explained her hears that the seizures, which are increasing in severity and frequency, could be causing Ben further brain damage:
“We have tried him on dozens of anti-epileptic medications over the years and they have failed to work.
“I see the fear in his eyes when he has the seizures. It breaks my heart. He can have anything between 250 and 300 fits a day.
“Some children with uncontrolled epilepsy are getting cannabis and others aren’t.
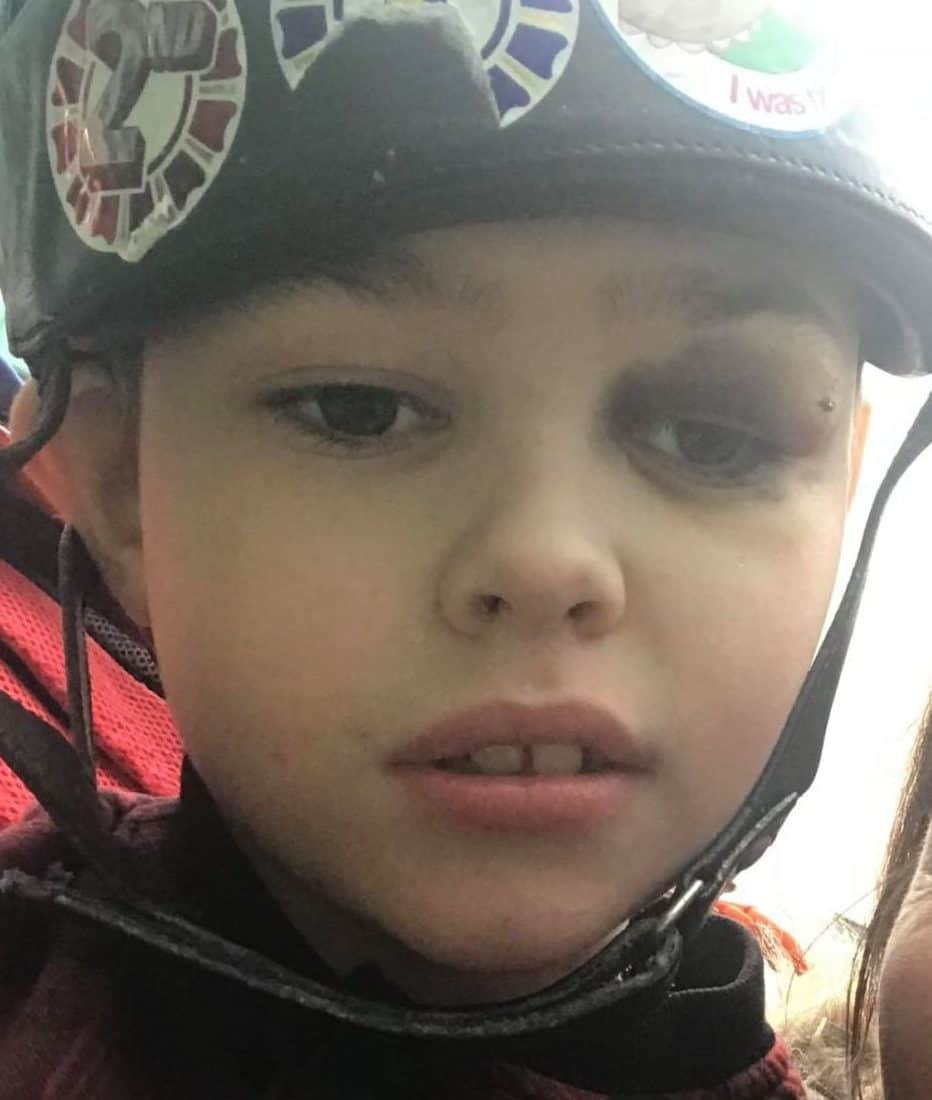
“There is clear clinical need for this for Ben yet Alder Hey are blocking its own consultant’s wishes.”
“We never stop worrying about him falling and hurting himself.
“We have bean bags all around the house and we cover the cabinets and sharp edges with bed sheets. But we can’t totally eliminate the risks for him.
“He’s a happy boy who loves life and the water but he’s had a fit while swimming at school and bumped his head.
“He can’t do these things that normal kids do anymore because he could die.
“We had spent the whole festive period cooped up at home so we thought we’d get out of the house and give the kids a break with a dog walk.
“Now Ben can’t walk or go anywhere because he has a fit every minute or two. If he does get up, he will hit the floor.
“He was screaming hysterically while fitting so we had to take him to hospital. His condition is very bad.”
“Some children with uncontrolled epilepsy are getting cannabis and others aren’t. There is clear clinical need for this for Ben yet Alder Hey are blocking its own consultant’s wishes.”
– Joanne Griffiths, Medical Cannabis Warrior
Joanne believes that while Ben’s current Epidolex prescription, containing only CBD and a trace amount of THC, initially managed to bring down the number of seizures, he needs a greater amount of the psychoactive cannabinoid:
“The Epidiolexis had a beneficial effect and dramatically reduced his seizures down from around 80 to 100 a day at the time to one to 20 a day.
“But then it stopped being as effective. His doctors then wanted to try him on a larger dose of an anti-epileptic drug we’d tried before which I was happy to give a go.
“But his fits got even worse and rose to up to 300 a day and they reduced this medication.
“We now want him to try Tilray and Bedrocan which come in higher THC form. Ben has already shown to be responsive to cannabis.
“It may now be the case that we need to find the right type – some THC along with CBD – and dose but we are being denied the chance.”
Joanne added that parents of children with severely uncontrolled epilepsy should not be put in a position where they are forced to fight for access to cannabis medication containing THC:
“Alder Hey say that the effectiveness of cannabis isn’t proven but it’s clear it’s helping kids like Charlotte Caldwell’s son so how can they say it won’t help my Ben when nothing else is working?
“They don’t know the long-term effects of THC on children’s brains but when the risk that their illness will kill them is greater it should be prescribed.
“That argument doesn’t even apply in our case as Ben has the mental capacity of a ten-month-old baby from his cerebral palsy and we’ve been told he won’t develop any further.
“We need the cannabis to control his fits and keep him alive.”
The family have requested a second opinion and to be referred to a neurologist in the Netherlands.
A spokesperson for Alder Hey Children’s NHS Foundation Trust said the trust cannot comment on individual cases:
“Neurologists at Alder Hey will consider whether a child is eligible to take cannabis-based medical products taking into account a number of factors.
“This includes the clinical history of the child, the scientific and clinical evidence for use of cannabis based medical products in particular clinical situations, and the published guidance from NHS England and the British Paediatric Neurology Association (BPNA).
“Alder Hey always works closely with families to discuss treatment options.”
Despite an increasing amount of clinical research which supports the use of medical cannabis for conditions like Ben’s, a stigma still exists amongst medical professional against cannabis.
Even with a prescription, patients are still being denied vital access to a life-saving drug, simply because of its source: cannabis.
While children in America and Spain are being treated with medical cannabis, with great success, British patients are being denied access.
Is Joanne right? Is it not fair to deny her child cannabis when others benefit?
MPs criticise NHS over failure to prescribe medical cannabis
MPs criticise NHS over failure to prescribe medical cannabis
- MPs have condemned the British Government for failing to provide medical cannabis to desperate patients
- Patients currently must have costly, private prescriptions in order to access legal medical cannabis
- Jorja Emerson’s family has to pay almost £10,000 a year (£833.75 per month) for her medical cannabis prescription for intractable epilepsy
MPs have condemned a “serious cultural block within the NHS around medical cannabis.”
The all-party parliamentary group for medical cannabis (AAPG) denounced the current procedure for acquiring a prescription for medical cannabis after it emerged that Jorja Emerson (the first child to be prescribed medical cannabis) must pay nearly £10,000 a year for their private prescription.
The AAPG claim that the Government cannot have envisaged the existing process when they voted to legalise medical cannabis in November. Mike Penning MP, co-chair of the AAPG said:
“For Jorja and her family this is a great day and I commend the private consultant, supported by her private hospital, who has taken the courageous decision to prescribe a medical cannabis product which she genuinely feels is in the best interest of her patient.
“But the fact remains that there is a serious cultural block within the NHS around medical cannabis.
“Other families, many with children just as seriously ill as Jorja, continue to face a complete block from their NHS medical teams.”
The Conservative MP for Hemel Hempstead raised the point that most families simply do not have the resources to afford private prescriptions, as in many cases:
“I do not believe that this is what the prime minister and home secretary envisaged when they made the bold change in the law on 1 November to reschedule medical cannabis to enable consultants to prescribe it.
“The number of even private clinicians willing to prescribe appears to be in low single figures”
“Denying parents the right for their children to try these newly available medicines is cruel.“
– Tonia Antoniazzi MP, co-chair AAPG
Tonia Antoniazzi MP, co-chair, joined the call for action, demanding a change to a system which fundamentally lets patients down:
“The high-profile cases over the summer of Alfie Dingley and others clearly show that for some epileptic children medical cannabis containing low concentrations of THC can be life-transforming.
“Denying parents the right for their children to try these newly available medicines is cruel.
“It appears that the medical profession is totally wedded to only prescribing a substance for which there is double-blind trial evidence. But there is a need for a common-sense perspective here.”
Antoniazzi also argued that some forms of epilepsy are resistant to conventional, pharmaceutical anti-epileptic drugs, often carrying severe, life-threatening side-effects, while medical cannabis offers a potentially safer alternative for patients:
“To our knowledge, no one has ever died from taking THC, and in any case the concentrations that we are talking about in these medicines are very low.”
Jorja Emerson, 2, suffers from a rare form of intractable, treatment-resistant epilepsy which causes her to suffer from life-threatening seizures. Pharmaceutical medication failed to ease the severity or decrease the number of seizures she was suffering, potentially causing permanent damage to the young girl’s body and brain.
Doctors have warned her parents that she might suffer a fatal seizure if her condition does not improve.
Speaking to The Guardian, Robin Emerson, her father, said:
“This has been an assault course of bureaucracy and a rollercoaster ride of severe ups and downs.
“I have faced near-total opposition in my quest to get my daughter access to a medicine that is now legal in the UK and has been shown to work in similar cases.
“No family should have to endure what we have been through.
“Thanks to the generosity of friends and supporters I have managed to raise enough money to fund the first few months of the medicine. But at £833.75 per month I face a desperately difficult future.”
Private prescriptions for medical cannabis are pricing-out patients who are desperate for an alternative to dangerous, addictive pharmaceutical medication.
Although cannabis was legalised for medical use at the end of 2018, few are benefitting from the change in law. Hopefully 2019 will see a change to the cultural fear of prescribing cannabis, allowing greater access to those who need it most.